Physics Over the Sink: Water Glass Magic

Defying the laws of gravity? Drinking water upside down? This must be magic… or science!
What you Need
- A glass
- Water
- A note card
- A sink

What to Do
- Fill your glass of water to the top.

- Place the note card over the top of the glass.

- Quickly turn the glass upside down over the sink (just in case the experiment goes wrong), holding the note card in place and then, carefully let go of the note card.

What’s Going On?
There are a couple of concepts at work in this experiment. The first has to deal with pressure and the second has to do with surface tension.
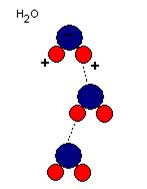
Diagram showing the positive hydrogen ends of the water molecule being attracted to the negative oxygen ends of the water molecule. Attraction represented by the dotted line.
There are a couple places where pressure is pushing against the card. First the atmospheric pressure or the tiny air molecules all around us are randomly colliding with the bottom of the note card. This pressure holds the card up, but why doesn’t the weight of the water push the note card down? Isn’t the weight of the water enough to overcome the atmospheric pressure pushing against the card? That’s what most people would think, but if you look at the top of the glass of water (formerly the bottom), you’ll notice a small pocket of air. Actually there isn’t much of an opportunity for air to get into that space, so what we’ve created is a small pocket of low pressure (a place where there really isn’t that many air molecules). There are more air molecules pushing up against the bottom of the note card, creating a higher pressure area compared with the lower pressure area inside the air pocket in the glass. The force from the atmospheric pressure holds the card up and the low pressure zone in the glass prevents the water’s weight from pushing the card down.
This is a great explanation of why this works, but there is more. The second concept in this experiment is surface tension and adhesion. Surface tension is created by how the water molecules orient themselves. Because the Oxygen atom steals electrons from the hydrogen atoms in a water molecule, water molecules become a dipole, in this case a molecule that has a positive end and a negative end. If you think about each water molecule like it is a tiny magnet, where like charges repel each other and unlike charges attract, you’ll see that there is a small attractive force between each water molecule. This force is responsible for surface tension, where the water molecule stick together through this attractive force, creating an elastic-like surface layer.
Adhesion occurs because water molecules, having the positive and negative ends, are also attracted to other materials. In the experiment that you conducted, the water molecules are attracted to the paper, adhering to it, while continuing to keep the surface tension with the other water molecules. This keeps the note card in place.
In summary, the note card doesn’t fall down because of the difference in pressure and it doesn’t slide off because of surface tension and adhesion. So, it might look like magic, but it’s really science.
Try This!
- Try putting soap around the rim of the glass and repeating this experiment. Make sure you do this over the sink. What happens?
- Try putting a small piece of mesh screen (like from a screen door) between the note card and the glass. What happens?











