Physics When It’s Cold Outside: Snowflake Symmetry

Bring the beauty of snow inside, while leaving the cold outside.
What you Need
- Sheets of white paper
- A scissors
What to Do
- Create a square sheet of paper from the 8.5 x 11 paper.
- Fold the sheet of paper diagonally like this:

- Cut off the rectangular piece at the bottom:
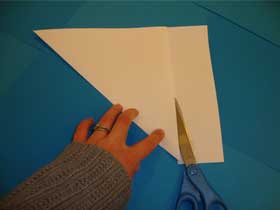

- Open the paper and you should see a square:
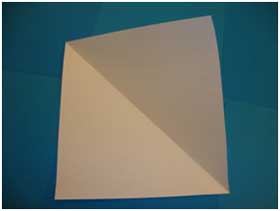
- Fold the sheet of paper diagonally like this:
- Create a hexagon. Watch the video tutorial:
- Refold your hexagon back into the triangle you previously created:



- Cut out shapes from the edges of the triangle:
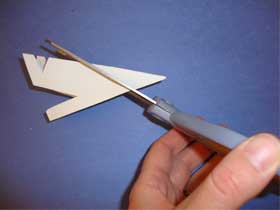
- Open up the triangle to reveal your hexagon shaped snowflake:
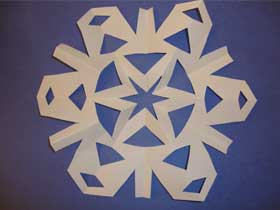
What’s Going On?
Patterns and symmetry are everywhere in nature. When you look at a leaf you see symmetry, where one half of the leaf looks like a mirror image of the other. This same symmetry often appears in a single snowflake as it clings to your mitten just before melting or blowing away.
How do snowflakes form? In clouds there are tiny molecules of water that are far apart, making a gas. When this happens with water molecules it is called water vapor. When the molecules in water vapor get close together they condense and start arranging themselves in repeating and symmetrical patterns, forming ice crystals. Different patterns emerge as these crystals grow. A snowflake is made up of one or more ice crystal. The water molecules in an ice crystal form a hexagon, a six sided structure (Figure 1). The hexagonal shape and symmetry of snow crystals comes from the hexagonal shape and symmetry of the ice crystal.
Water molecules are made up of two hydrogen atoms and one oxygen atom. The full hexagonal shape of an ice crystal is made up of 6 water molecules where only 6 hydrogen atoms are shared between the oxygen atoms, forming the hexagonal shape of the crystal.
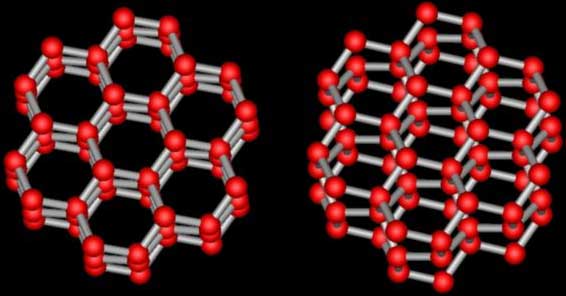
Figure 1:The hexagonal shape of an ice crystal from above (right) and another view showing the layers to be more spread out (right) . The red balls represent oxygen atoms and the gray sticks represent hydrogen atoms.
Try This!
- Experiment with cutting out different shapes. How does it affect your snowflake?
- Try folding your paper in different ways. How does this impact the symmetry of your snowflake?
More Information











