Physics in the Microwave: Microwave Soap
*Adult supervision recommended
What did people do before microwaves? Imagine having to use the oven to heat up your leftover pizza or an air popper to make popcorn…and how else could you make these amazing soap sculptures??
Warning
- Do not leave the microwave unattended during the activity.
- Although heating up soap in the microwave will not damage your microwave or the food you heat in it later, it will cause the microwave to smell like soap for a few hours.
- Do not place metal in the microwave.
What you Need
- Bar of soap (Ivory® works well)
- Microwave
- Paper plate / shallow plastic container
What to Do
- Place the soap on the plate (or shallow container) and put it in the microwave.
- Set the microwave for 2-3 minutes, turn it on, and watch the soap for the entire time. What happens? (soap in microwave)
- Let the soap cool for a few minutes and then take it out of the microwave. What does it feel like? Break off a piece and run it under water. Does it still act like soap?
- Be sure to wipe out the microwave when you’re done!
What’s Going On?
The “microwave” part of “microwave oven” actually refers to how your food (or whatever else you put inside) is heated up in the microwave oven. In a previous experiment we talked about how infrared light is part of the electromagnetic spectrum and has slightly longer wavelengths than visible light (red, blue, etc). Microwaves are another type of light – and have even longer wavelengths than infrared light.
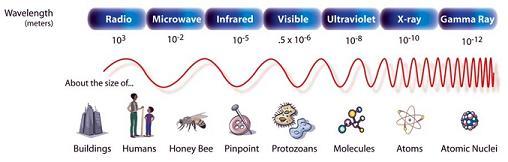
This electromagnet spectrum shows all the different kinds of light in order from the longest wavelength to the shortest. Image courtesy NASA.
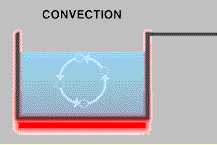
Microwave ovens send microwaves waves back and forth through your food. This causes the water, fat, and sugar molecules in the food to rotate and bump into each other, which produces heat. This is different than heating food in an oven or on a stove, which are both based on heating by convection.
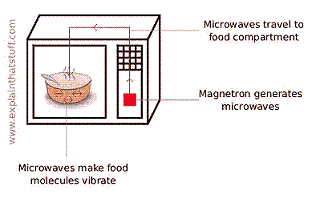
Left: Microwaves are created inside a microwave oven. As they travel back and forth through the food (or soap) in the microwave, they cause some types of molecules to rotate and hit each other. This produces heat. Image courtesy Explain that Stuff!
Right: A pot of water on the stove heats by convection. The burner heats the water closest to it – making the bottom layer of water warmer than the rest of the water. But cold water is denser than warm water, so it sinks to the bottom and forces the warm water up to the top of the pan. This water is heated by the burner until it is hotter than the water above it, and the cycle keeps repeating. Image courtesy NOAA.
Popcorn “pops” in the microwave because the water molecules inside the kernel turn to steam as the kernel heats up. This increases the pressure inside the kernel, eventually leading to an explosion when the outer layer of the kernel can’t stand up to the pressure anymore. Soap expands for a similar reason.

Popcorn before and after it pops.
Soap contains small pockets of air that have water vapor trapped inside of them. Like the moisture inside of a popcorn kernel, the water vapor inside of soap heats up when the microwave is turned on. This leads to areas of high pressure inside the soap. Eventually the softened “walls” of the bar can’t hold up to the pressure and the soap starts to bubble and expand. As the soap cools down it stiffens up but keeps its new shape.
Try This!
- Place Ivory soap and some other brands of bar soap in a sink full of water. Which ones float? What does this tell you about how much air is trapped in each brand of soap?
- Heat up other brands of soap in the microwave. Which ones expand the most?
- Carefully heat a bar of soap in a pan on the stove. What happens? How does this compare to what happened when you heated the soap in the microwave?
More Information











