Seeing Atoms
What does it mean to see an atom?
Suppose you tried to use the world’s strongest optical microscope to see an atom. What would happen? You would probably reflect light from the atoms into your microscope. Light has wave properties, so imagine waves of light shining on an atom. The wavelength of visible light is about ten thousand times the length of a typical atom.
 To help think about this, let’s switch to thinking about water waves rolling in on a beach. If you stand in the water, the waves roll past you, unaffected. Hardly any wave energy is reflected. That’s because the size of your body is so much less than the wavelength of the water waves. The waves move by as if you were not there, so reflection of water waves will not reveal the presence of a person in the water.
To help think about this, let’s switch to thinking about water waves rolling in on a beach. If you stand in the water, the waves roll past you, unaffected. Hardly any wave energy is reflected. That’s because the size of your body is so much less than the wavelength of the water waves. The waves move by as if you were not there, so reflection of water waves will not reveal the presence of a person in the water.
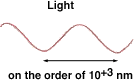
The wavelength of visible light is about 10-6 m (the same as 103 nm), as shown in the drawing . The size of a typical atom is about 10-10 m, which is 10,000 times smaller than the wavelength of light. Since an atom is so much smaller than the wavelength of visible light, it’s much too small to change the way light is reflected, so observing an atom with an optical microscope will not work.
How about radiation like light but with a shorter wavelength? X-ray wavelengths are about the same size as atoms, but reflecting x-rays from matter forms a complex pattern of spots that depends on the arrangement of the atoms. Analysis of these patterns reveals a lot of important information about crystals, but the x-ray images do not show individual atoms.
Research
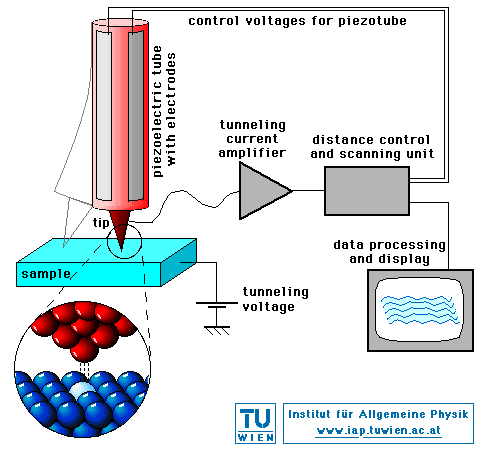
The best way to image atoms is with a device called a scanning tunneling microscope. It is based on tunneling, a quantum-mechanical effect roughly analogous to water leaking right through the sides of a glass. If a small needle comes within about 10-9 m of a metal surface, an electric current, due to tunneling, starts to flow. The size of this current depends upon the separation of the needle and the atom and decreases as the separation increases. (See diagram.)
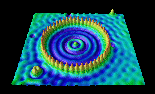
A corral made by placing 48 iron atoms in a circle, one at a time, onto a surface of gold (Reprinted with permission from IBM)
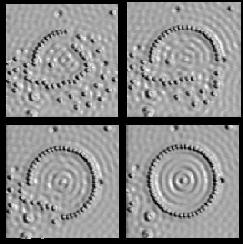
Several stages in the assembly of the quantum corral (Reprinted with permission from IBM)
An automatic control system based on this current produces a detailed map of the surface. If the current starts to go down, the needle is moved towards the atoms to bring the current back up, and vice versa. Monitoring the movement of the needle makes an image of the surface, right down to the size of individual atoms.
The image shows what is called the “Quantum Corral.” A ring of 48 iron atoms corrals electrons, shown in blue, inside the ring and prevents them from escaping. The ripples inside the ring display the wave properties of electrons.
How do you move an atom? It turns out that the atom will stick to the tip of the needle if the current is just right. When the atom is in the desired position, the current is reversed and the atom remains in place on the surface. The image shows the quantum corral being assembled.
This technique makes possible a whole new kind of chemistry investigation, where chemical reactions are studied in exquisite detail by bringing together atoms to make molecules one at a time. After the reaction, the molecule remains on the surface, where it is easy to observe with the scanning tunneling microscope.
Links
IAP/TU Wien
IBM
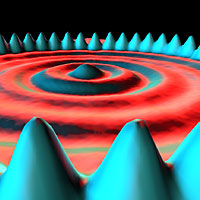
Another view of the circular quantum corral of iron atoms (Reprinted with permission from IBM)