Ultrafast Aluminum Battery
June 9, 2015
What doesn't this battery have? It’s flexible, fast, nontoxic, doesn’t catch on fire, and its materials are inexpensive. It can hold the same charge even after 7500 recharging events, compared to between 1000 and 3000 for Lithium ion batteries. It can reliably deliver between 2 and 2.5 V. While it's got its limitations, the list of benefits this technology could soon offer goes on and on.
Some History
Before 1800, electricity was produced by friction – rubbing amber with cloth would transfer electrons from the cloth to the amber, making the amber negatively charged and the cloth positively charged. Rubbing glass with cloth would make the glass positively charged and the cloth negatively charged. Benjamin Franklin assigned the terms positive and negative in the 1700s.
In the 1780s Luigi Galvani, a professor in Italy, was doing experiments on the contraction of muscles using electricity. He found that the muscles of a frog’s leg contracted when an electrically charged object touched the muscle. He also found that if he placed two different metals in the muscle, (no charge applied) the muscle contracted. Many people of the time thought this was the “life-force.”
Volta, a professor at a nearby university, was skeptical and decided to test how different metals interacted with the frog muscle and came to the conclusion that the frog muscle was a far better device for measuring what today we call voltage (a measure of potential energy per charge) than the measuring device used at the time. He also suspected that perhaps it was the moisture in the frog’s leg that allowed the charge to flow between two different metals, and he was correct. He tested many metals and types of fluid and found that some produced a larger effect than others. Volta decided to stack discs of different metals with a cloth soaked in a salt solution or a cloth soaked in a dilute acid solution, the two solutions he found to produce the largest effect. When a thin strip of wire nearly connected two stacks, sparks would fly. Volta’s creation was the first battery, and is now known as a pile battery.
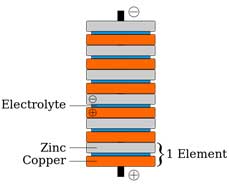
Diagram of Volta’s pile with felt soaked in sea water between the copper and zinc discs. "Voltaic pile" by Borbrav, svg version by Luigi Chiesa
Image credit: Voltaic pile.png. Licensed under CC BY-SA 3.0 via Wikimedia Commons
A zinc ion (Zn2+) goes into the electrolyte (the salt water solution), which is called oxidation because the zinc is leaving electrons behind and will form zinc oxide (ZnO) with oxygen atoms in the solution. The zinc disc becomes negatively charged. Two hydrogen ions (H+) from the water acquire electrons from the copper to form H2 gas that bubbles away (this is called reduction because electrons are acquired). The copper discs are left with a positive charge. Electrons from the zinc discs flow to the copper discs.

One of Volta’s pile batteries. "VoltaBattery" by I, GuidoB.
Image credit: Licensed under CC BY-SA 3.0 via Wikimedia Commons
Basic Battery Physics
The basic physics behind a battery is simple. Like charges repel, unlike charges attract. If one can get a bunch of like charges in one location they will repel each other and spread out, given the chance. It turns out to be easiest to work with the outermost electrons of metals. These flow very well from one metal atom to another and, to distance themselves as much as possible from each other, they spread out across the outer surface of the metal object. If the charged metal object is connected to a less negative, neutral, or positively charged object that allows electrons to flow, the electrons from the negatively charged metal object will get pushed by the repulsive force between them to the neutral or less negative area, with an additional pull if there are positive charges on the other object. The anode is defined as the source of electrons, and the cathode is the side the electrons flow to when the battery is discharging. Current (the amount charge per time that flows past a surface) is defined to flow in the opposite direction of electrons for historical reasons.
Along the way, these energy carrying charges can give up some of their energy to a device like a light bulb, a warming coil in a toaster, or a laptop.
The creation of positive and negative terminals is just what Volta did. In regular dry cell or alkaline batteries, rather than have a liquid solution, a paste is placed between the different metals that has enough moisture in it to allow the ions to move into solution to create an anode and cathode. The outside terminals of the battery are connected to the different metals inside the battery.
Three important ingredients are: 1) A solution that allows the ions to be formed and transported to create the anode and cathode. 2) A conducting material that will interact with the solution so some of its atoms to go as ions into the solution, leaving electrons behind. 3) A second material that will interact with the solution or positive ions so that it becomes positively charged, stays neutral, or does not get as negatively charged as the first material. It must also allow the flow of electrons over it.
Chemical reactions power the battery, and also cause it to decay over time as buildup of material occurs on the different metals, or as the solution breaks down due to reactions not involved with the formation of the negative and positive terminals. Eventually the battery “dies” as it becomes unable to produce a charge on the two terminals. For rechargeable batteries this degradation is slow and the chemical reactions that occur can be reversed many times before the degradation of materials affects the battery.
When materials used for creating the charges on the cathode don’t allow electrons to flow easily, carbon is often added to assist in the flow of the electric charge during discharge (and recharging, if it is a rechargeable battery).
What’s New With Aluminum
Aluminum batteries have aluminum as the negative terminal or anode. They have long been sought since aluminum is known to be able to give up three electrons, which is a lot of charge per ion going into solution. However aluminum batteries have never before performed well. Although they produce a good charge, the cathodes would rapidly degrade causing low voltages and short lifetimes.
A research team has found a way to fix that problem. Their novelty is two-fold: the selection of their solution (made of an ionic solution containing aluminum trichloride 1-ethyl-3methyllimidazolium chloride) and the material structure (rather than the material type) of the cathode. Instead of using your typical graphite (carbon), they used a graphitic foam.
First we consider, as the experimenters did, pyrolytic graphite, which is made of very organized sheets of carbon molecules layered on top of each other, with some covalent bonding between layers.
The aluminum reacts with the solution in the following way:
![]()
The ions in solution become embedded between the layers of carbon molecules, a process called intercalating
![]()
This is how things are set before connecting the two terminals.
The carbon cathode interlaced with AlCl4 molecules is not positively charged, but provides a good place for the electrons to get pushed to. When the terminals of the battery are connected, the electrons will flow from the negatively charged aluminum anode to the cathode. The carbon will let the electrons flow, which hook up with an AlCl4 molecule to form an AlCl4¯ ion that can go back into solution. This is called de-intercalation.
When an aluminum sheet (anode) is connected to graphite layers (cathode), electrons from the anode freely flow to the cathode, reversing the intercalation reaction given above.
![]()
The scientists found that sometimes the AlCl4¯ ions, which are about 52.8 billionths of a meter across, get stuck between the layers of graphite which are spaced about 57 billionths of a meter, affecting the recharging. So they decided to try a novel structure. They created a foam base and coated it with graphite “whiskers”. An image of it taken with a scanning tunneling microscope is shown below. The foam allows better intercalation and de-intercalation.

Scanning electron microscope image of the graphitic foam.
Image credit: Bingan Lu
A few cool things about this battery are that it can completely discharge in less than 60 s or gradually over 1.5 hours for the pyrolytic graphite, and 34 minutes for the foam. The battery can be recharged in under 60 seconds more than 7500 times to within 98% of its full capacity for the pyrolytic graphite, and 100% of its capacity for the foam! This recharge capability is much better than the lithium ion battery in your phone, which takes a while to charge, can only be effectively recharged between 1000 to 3000 times, and never so close to its initial capacity. Another feature is that the batteries are flexible packs that can be bent without damaging them. They also do not catch fire, which has sometimes been a concern with lithium batteries. However, it is important to note that there are also limitations.
Some Limitations
This new technology has limitations that might be overcome in the future, but certainly won’t prevent it from being utilized in the present. It has an optimal voltage range between 2 and 2.5 volts, where lithium ion batteries reach about 3.5 volts. That means a series of aluminum batteries would be needed for voltages over 2.5 volts.
Another important measure of a battery is how much energy it can store per gram. It turns out that, per gram, the new aluminum battery can only store about ¼ the energy that a lithium ion battery can store, so battery packs for phones, computers, or cars might become a bit unwieldy.
Below is a video showing the new battery and some of its features.
Future Research and Applications
It has already been noted that these batteries might be very useful in the temporary storage and rapid release of energy on the grid to assure a continuous and steady flow of energy. The fact that these batteries can be charged and discharged so rapidly, and that recharging can occur so many times makes them ideal for this type of use.
References and Resources
1. Lin, M-C. et al., “An ultrafast rechargeable aluminum-ion battery,” Nature 520, 324–328 (16 April 2015) doi:10.1038/nature14340
http://www.nature.com/nature/journal/v520/n7547/full/nature14340.html
2. Schwartz, M. Aluminum battery from Stanford offers safe alternative to conventional batteries, Stanford News 6 April 2015.
http://news.stanford.edu/news/2015/march/aluminum-ion-battery-033115.html
3. Fares, R., Stanford Researchers Unveil New Ultrafast Charging Aluminum-Ion Battery, Scientific American Blogs, 14 April 2015.
http://blogs.scientificamerican.com/plugged-in/stanford-researchers-unveil-new-ultrafast-charging-aluminum-ion-battery/
4. Giancoli, D. Physics, Principles with Applications 7th edition, Pearson Prentice Hall, Boston, 2014.