"Breathprint" Identification
June 6, 2013
We can already use fingerprints and DNA to identify individuals, but an incredibly fast and noninvasive technique may be able to uniquely pick out people based on their breath!
Individualized Medicine
Individualized medicine involves investigating a person’s genome, cellular activities, and inner environment to tailor strategies for prevention, detection, and treatment of disease, and it is becoming more popular. Urine tests have already suggested persistent unique information for individualized medicine, providing information on cellular activity and environmental factors within the body.
This led a group of researchers in Switzerland to look into information contained in human breath. They found significant information for individualized medicine, and a unique set of data for each individual they tested! They found a breathprint.
For example, if ten people were to drink a cup of coffee, their breath (and urine) would show signs of how their body interacted with the caffeine in the coffee. Perhaps some interacted the same way, perhaps not, but the data would give insight into how the individual interacts with caffeine.
However, if every individual interacted differently with the caffeine in a measurable way, then the caffeine might be considered as an identifier like a fingerprint.
Individualized medicine requires knowing how your human cells work and what environment they are in. Did you know that for every human cell in your body there are at least ten non-human cells in there? Really, those microbes in there make you less human than you think!
Doctors working in individualized medicine also investigate an individual’s phenotype, a composite of genetic and environmental factors. This can all be considered in metabolomic studies, which are studies of chemical processes during cellular interactions. Your breath, particularly the last part of what you exhale, contains information on these interactions.
For this research, the scientists analyzed 11 people’s breath four times a day over a nine day period. For each of the four data collection time slots during the day, the subjects abstained from eating, drinking, and brushing their teeth for half an hour before the test, and then they breathed steadily, three times, into a tube.1

An image of a subject breathing into a mass spectrometer overlaid with mass spectrometry data used to identify individuals.
Image Credit: Xue Li / Christine Khammash / ETH Zürich
Breath Analysis
The process is non-invasive and the analysis doesn’t take that long. So how do they do it? Well, that tube the subjects breathe into is connected to a mass spectrometer, an instrument used to separate particles that have different mass to charge ratios. The breath from the tube first enters a gas port (a chamber) where it encounters a charged mist of water mixed with formic acid.1 In the chamber, the droplets and the molecules of breath mix.
As this mix continues to travel in the gas port, the charged droplets begin to evaporate, resulting in the surface area of the droplet getting smaller, and the charge on the surface getting closer. Eventually the charged droplet is small enough that the force between charges overcomes the surface tension of the drop breaks apart. This process continues until only ionized (charged) molecules from the breath sample remain. These then continue into the mass spectrometer where they will be separated according to their mass to charge ratios.
One way to understand how mass spectrometers work is to consider a few applicable physics principles. Mass spectrometers make use of the interaction between the charge on a moving particle with an electric and/or a perpendicular magnetic field, which provides a push or pull on the charged particle. The greater the charge or the field, the greater the overall force on the particle.
Force = mass * acceleration = charge(electric field + velocity x perpendicular magnetic field)
The second physics principle used is Newton’s Laws, and it deals with how this push or pull accelerated the particle. If the charges are all equal, then the less massive particles will accelerate the most. (Note, one might also use an energy approach to explain mass spectrometers.)
One of the simplest types of mass spectrometers uses the interaction between charged particles and magnetic fields. If a moving charge goes through a magnetic field that is not aligned with its direction of motion, then it feels a force perpendicular to both the magnetic field and the direction it is moving in.
For example, if a positively charged particle is moving upward, and the magnetic field points out of the page, then the charged particle would feel a force to the right, and begin to turn in that direction. The acceleration (change in speed and/or direction) an object experiences is the ratio of the overall force exerted on the object and the mass of the object.
For this simple spectrometer, if two particles have the same charge and different masses, the smaller mass will accelerate more than the larger mass, resulting in a tighter turn to the right, as shown in the diagram below.
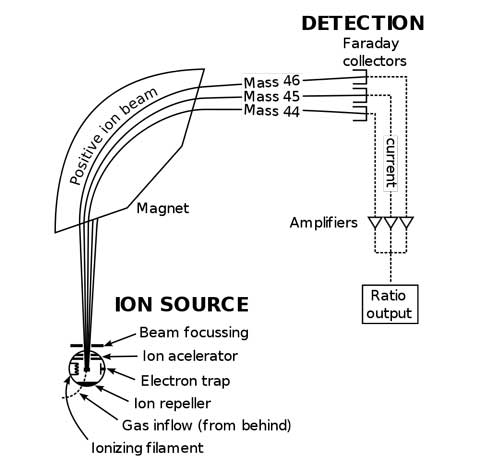
Simplified version of a mass spectrometer. The charge is the same for the three particles shown. The heavier mass (Mass 46) is deflected less than the lighter mass (Mass 44).
Image Credit: Devon Fyson
The spectrometer used in this experiment is a quadrupole time-of-flight (QTOF) spectrometer. The first term of this spectrometer, “quadrupole,” means that the charged breath particles go through a special filter that only allows a specfic selected range of mass to charge ratio to pass through. The filter is an oscillating electromagnetic field (in the radio frequency range) and a constant field, created by four conducting rods.

Photo of mass spectrometer quadrupole.
Image Credit: Fulvio314
The filter setting for mass to charge ratio can be tuned as quickly as your radio. The moving charged particles interact with the fields. They end up being pushed back and forth and move forward between the four rods.
If the mass to charge ratio is just right, the particle will get pushed back and forth (oscillate) in resonance with the field frequency chosen, and get pushed forward (accelerated) into further parts of the spectrometer. If the mass to charge ratio is not just right, the particle will get pushed out of the filter, and not continue into the rest of the spectrometer.
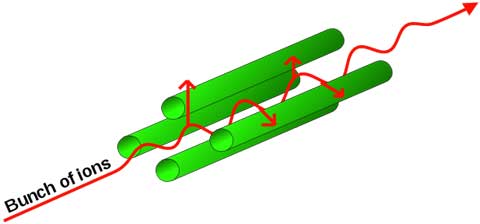
Diagram of charged particle in resonance traversing quadrupole.
Image Credit: Angelus
The accelerated, filtered particles then enter the field-free time-of-flight part of the spectrometer. Here the particles travel undisturbed. All the particles that made it this far have the same mass to charge ratio.
The least massive particles gained the greatest speeds before entering the time-of-flight region, and the most massive particles have the slowest speeds. Detection of the particles occurs at the end of this time-of-flight region; therefore, the least massive particles are detected first, and the most massive particles are detected last. Using this data, researchers can determine which molecules are present.
76 Percent Recognition
Zenobi’s team only considered the last 6 seconds of breath – this contains the most information from deep down in the lungs. The team measured 10,000 m/z (mass to charge) values.1 They then put some constraints on what data they would consider: They only considered mass to charge ratios that were significantly above noise levels and the differences between one subject needed to be significantly different from another subject. With these constraints their data set reduced to 2,928 mass to charge ratio values.1
After considerable analysis utilizing standard methods1, the researchers plotted their results on just three of the ten dimensions involved in the analysis. If you click the link to look at the colorful, three-dimensional plotted data in their paper, you will see that these three dimensions show each individual’s data clusters in a certain region.
It is important to note that for some of the individuals they collected data on, they did not have a complete data set.
Their individual correct classification rate was between 62% to 92%1 which included incomplete data sets, and different time slots for measurements. The overall recognition score was 76%. Although this is not 100%, it is still a significant find and indicates more studies should be done. Who knows, perhaps future passwords might be a combination of recognizing fingerprints, eye scans, and breath!
The Road Ahead
Uniquely identifying a patient’s breath may prove particularly useful in the growing field of individualized medicine. Additionally, using this as a means of identification may prove useful outside of medicine. These studies will continue, and as the data comes in, more applications may emerge. For now, researchers remain focused on the medical applications.
When asked what he would like to see happen with this research, Dr. Zenobi said, “I would like to see the development of small, perhaps even field-portable, mass spectrometers with sufficient sensitivity, robustness, and accuracy such that these analyses could be run in a clinic or doctor's office.”
References and Resources
1. Martinez-Lozano Sinues, P., Kohler, M., & Zenobi, R. (2013) Human Breath Analysis May Support the Existence of Individual Metabolic Phenotypes, PLoS ONE 8(4): e59909. Doi:10.1371/journal.pone.0059909http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0059909
2. Summers, R., What we exhale is unique to us – our ‘breathprint’, New Scientist, 4 April 2013.
http://www.newscientist.com/article/dn23347-what-we-exhale-is-unique-to-us--our-breathprint.html
3. Meier, C., Recording a breathprint, ETH Life international, 12 July 2006
http://archiv.ethlife.ethz.ch/e/articles/sciencelife/atemluftzenobi.html
4. Mass Spectrometry, Wikipedia
http://en.wikipedia.org/wiki/Mass_spectrometry
—Heide Doss