Bullet Proof: Absorbing Micro-Bullets
Polyurethane, a common type of “bullet-proof glass,” can stop a speeding bullet and reseal itself after impact. Determined scientists have finally gained more insight into how polyurethane is able to dissipate so much energy without sustaining more damage.
To unravel this mystery, a research team created a model consisting of microscopic glass bullets, a firing mechanism, and a nano-layered, bullet-proof target material that mimicked polyurethane. The nano-layered composite target material captured speeding glass micro-bullets and resealed itself!1 This microscopic system has elucidated the mechanisms behind polyurethane’s strengths and has provided potential for significant new applications.
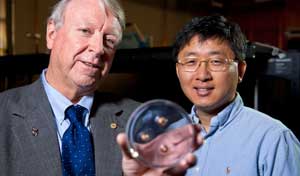

Figure 1. Left: Researchers Ned Thomas and Jae-Hwang Lee of Rice University holding a polyurethane disk with bullets embedded in it. Right: A close-up of the polyurethane disk with embedded bullets.
Image Credit: Rice University/Tommy LaVergne
Building Bullet-Proof
Three and a half centimeters of polyurethane can arrest a bullet traveling at 350 meters per second.1 It has glassy (more rigid) and rubbery (more stretchy) areas on the nanoscale, and it can “heal” itself after a bullet penetrates its surface. Researchers already knew what polyurethane could do; they just didn’t know how and why.
To gain insight, they searched for glassy and rubbery materials they could layer in a controlled way. Two candidates made the cut.
Polystyrene (PS) is glassy. It is rigid and is used to make DVD cases, plastic utensils, and bottles. Polydimethylsiloxane (PDMS) has rubbery properties. Its elasticity makes it useful for contact lenses, caulking materials, and shampoo.
Both materials are polymer chains, have a crystalline structure, and one (PS) is more rigid than the other (PDMS). Alternating layers of these materials is called polystyrene-polydimethylsiloxane diblock-copolymer or PS-b-PDMS diblock-copolymer. A polymer is a chain of molecules with a repeating molecular structure.
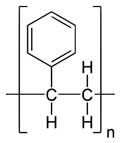
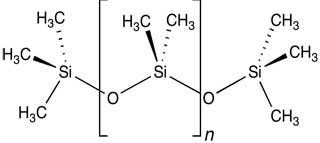
Figure 2. The mer in polymer is the molecular structure that repeats itself; [mer]n = poly mer. Left: Polystyrene, PS, or [C8H8]n. Right: Polydimethylsiloxane, PDMS or CH3[Si(CH3)2O]nSi(CH3)3.
Image credits: Yikrazuul and Smokefoot respectively via Wikimedia Commons.
The researchers deposited alternating layers, 5 to 20 nanometers thick, of glassy and rubbery materials, in a highly organized and regular way.1 Under a scanning electron microscope (SEM), one can observe the layers as a striped pattern.
Balancing Glassy and Rubbery
The temperature of an object describes the average kinetic energy (energy of motion) of the molecules and atoms that make up the object. An example of this is melting. Different substances melt at different temperatures and this is a property of the material. As the motion of the molecules increases, the bonds holding molecules together breaks, and one goes from a solid structure to a liquid structure.
Within the bullet-proof material’s layered structure, the two materials have very different transition temperatures -- the temperature at which the structure of the molecules changes. Changing molecular structure does not necessarily mean a change in phase (solid/liquid/gas). For polymers, the glassy transition indicates when solid polymer chains go from sliding past each other easily (rubbery) to where they become rigid (glassy). This transition is called the glass transition temperature.
This transition temperature is 103˚C (217.4˚F) for the rigid PS and -125˚C (-193˚F) for the rubbery PDMS at standard pressure. That’s a 230˚C (446˚F) difference! These transition temperatures increase as the pressure increases, like when a bullet strikes.
Their elastic properties are very different too. One important elastic property relates the ratio between how a material wants to restore itself when being pushed or pulled and how much it has changed its shape. This ratio is called the elastic modulus.
elastic modulus= stress/strain
- Stress = the force that the object tries to restore itself with per area
- Strain = the ratio of the deformed object to its original shape
For the rigid PS, the elastic modulus is 3.5 billion Pascals at standard pressure. The rubbery PDMS has an elastic modulus of about 750 thousand Pascals at standard pressure. This is a huge difference: PS is about 5000 times more rigid than the rubbery PDMS at room temperature. These elastic moduli also increase as the pressure increases.
Absorbing a Bullet
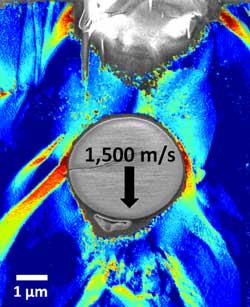
Figure 4. Scanning electron microscope image of a glass bullet, fired at 1,500 meters per second into a PS-b-PDMS diblock-copolymer.
Image Credit: Dr. Jae Hwang Lee, Rice University.
As a force deforms an object some of its molecules get pushed closer together and others get pulled further apart. This sets up vibrations rotations, and other jiggling motions within the molecules and between the molecules. The average motion energy of atoms represents an object’s temperature. Consequently, deformation increases the temperature of an object.
If the temperature gets as high as a transition temperature, then restructuring of the molecules or the chemical bonds can occur. If the temperature increases to a change in phase transition temperature, the crystalline structure of these molecules breaks down, and they slide over each other in a liquid state.
A fired bullet has a lot of energy associated with it.
As it interacts with the molecules making up the material it strikes, it transfers its energy to them. It pushes the molecules around, makes them move more, increases their temperature, and melts some of them. How well a material dissipates all of the bullet’s energy is the key to creating a “bulletproof material.” Polyurethane is good at dissipating a bullet’s energy. So is the PS-b-PDMS diblock-copolymer.
The researchers modeled the bullets with microscopic silica beads that were 3.7 microns (3.7 millionths of a meter) in diameter and watched what happened using a scanning electron microscope (SEM).1 What they saw is pictured on the left.
In front of this embedded glass bullet that was fired at 1,500 meters per second, you can see a cavity. Cavities often form just in front of the bullet because as the bullet transfers energy to the material, the bullet slows down more rapidly than the material. The layered material keeps moving forward, transferring energy to subsequent layers and then stops.1
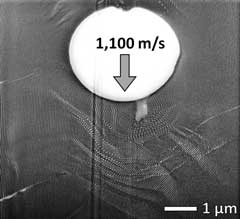
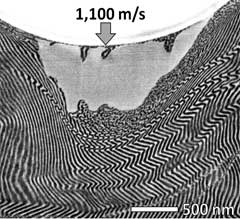
Figure 5. Left: Magnification of glass bullet fired at 1,100 meters per second into a PS-b-PDMS diblock-copolymer. Here you can see the kinking of the layers of the material. Right: Further magnification of the left image, showing regions where melting (uniform grey area) and mixing (granulated area) occurred.
Image Credit: Dr. Jae Hwang Lee, Rice University.
In the images above, you can see where the layers of the material were compressed, mixed, and formed cavities. As the bullet pushes the material forward, the layers of PD and PDMS get compressed in the forward direction and elongated on the sides.
Some areas melt, and the PD and PDMS polymer chains get mixed (the granular and uniform grey looking areas). Behind the bullet’s entry, the material fills in and heals the entry hole due to the elastic recovery of the material that was displaced by the bullet, and with the granular looking mix of PD and PDMS that melted.
Current and Future Research
Using their findings, the researchers have gained insights into how materials like polyurethane multiblock and PS-b-PDMS diblock-copolymer dissipate energy. They plan to use this information to develop new materials that could be used to protect satellites from impacts with micrometeorites, the blades of jet engines from impact with particles, and even body armor from bullets.
References and Links
1. Lee J.-H. et al., High strain rate deformation of layered nanocomposites, Nat. Commun. 3:1164 doi: 10.1038/ncomms2166 (2012).
http://www.nature.com/ncomms/journal/v3/n10/full/ncomms2166.html
2. Williams, M. Microbullets reveal material strengths, Current News, Featured Stories, 30 October 2012
http://news.rice.edu/2012/10/30/microbullets-reveal-material-strengths-2/
3. Chandler, D.L., Stronger than a speeding bullet, MIT News Office, 7 November 2012.
http://web.mit.edu/newsoffice/2012/bullet-stopping-nanostructured-material-1107.html
4. Ornes, S, How to stop a speeding bullet, Science News for Kids, 26 Nov 2012
http://www.sciencenewsforkids.org/2012/11/how-to-stop-a-speeding-bullet/
—H.M. Doss