The Little Constant that Couldn't?
Physicists measure the values of basic quantities like the speed of light and the charge of the electron. Cosmologists use the results in studies of the origin of the universe, some 12 billion years ago, and they assume the numbers have not changed over this time.
But now comes a result that could stand this assumption on its head. A research group claims that the fine structure constant, written as the Greek letter alpha (α), has increased over the last six billion years or so. Admittedly, the increase is only one part in 100,000-pretty small-but that's enough to be plenty unsettling.

Quasar spectra recorded at the Keck Observatory in Hawaii imply that a fundamental physical constant may have been increasing slightly over the past six billion years. (Image courtesy of NASA/JPL/Keck)
Alpha specifies how strongly electromagnetic waves (like light or x-rays) affect charged particles (like electrons and protons). Alpha is actually a dimensionless ratio-all units cancel out-involving three quantities:
- the charge on the electron
- the speed of light
- a fundamental constant from quantum mechanics
The idea that one or more of these quantities changes over time is generally most unappealing, although a few theories actually have suggested that it might happen. Since the observed effect is small, many physicists have decided to await further results before making any judgements.
The Measurement
The measurement of a is based on the way atoms absorb and emit light. An isolated atom can emit light in particular wavelengths, as shown in the upper part of the drawing. The frequencies of these lines are characteristic of each chemical element. In certain elements, one of these lines can be split. An example is the bright yellow pair of lines in sodium, responsible for the yellow color of sodium street lamps. The amount of this splitting is proportional to the fine-structure constant.
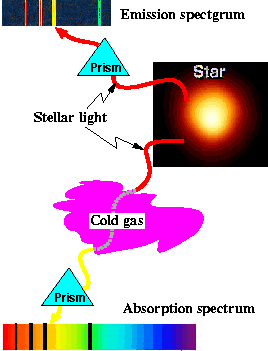
Formation of emission and absorption lines (drawing courtesy of Jose Wudka, University of California at Riverside)

A quasar and its host galaxy (Image courtesy of the Space Telescope Science Institute, operated by the Association of Universities for Research in Astronomy, Inc., from NASA contract NAS5-26555)
In addition to such emitted light, spectral lines are also observed in absorbed light. (See the lower part of the drawing.) When a continuous spectrum of light passes through a cool region of atoms, the atoms absorb out of the continuous spectrum the very same frequencies that these atoms can emit. Astronomers match such dark absorption lines to bright emission lines observed in the laboratory to identify the species of atoms doing the absorbing. (See drawing.)
Imagine that out in space there is a cool cloud of a gas characterized by the split spectral lines mentioned above. Passing a continuous spectrum of light through this gas produces two nearby absorption lines. Just as with the emission lines, the spacing between the absorption lines is proportional to the fine-structure constant.
Astronomers have identified quasars, extremely bright and distant objects, whose light passes through such cool gas clouds on its way to Earth. The separation in the absorption lines is slightly different from the separation measured in a laboratory on Earth, to about a part in 100,000. The gas clouds are extremely distant, so the interpretation of the results is that the value of the fine-structure constant has changed during the time required for the light to reach Earth.
More About the Fine Structure Constant
The fine structure constant, alpha (α), describes how electromagnetic radiation affects charged particles. It has the numerical value 0.007297351, with an uncertainty of 6 in the last decimal place, and as such is one of the best-measured numbers in physics.
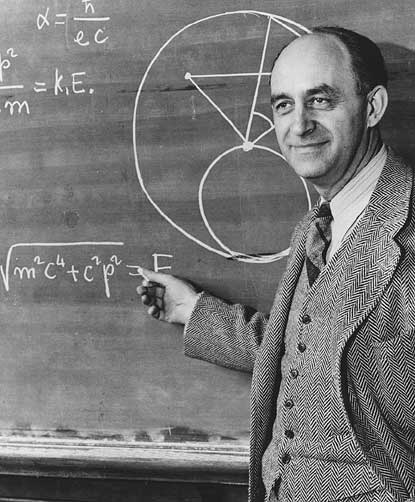
This picture of Enrico Fermi, appearing on a recently issued stamp, contains, in the upper left corner, an incorrect formula for alpha. Proving that even Nobel Laureates make mistakes… (Credit: University of Chicago, courtesy AIP Emilio Segre Visual Archives.)
Note that α is quite a small number, very nearly 1/137. This makes theoretical physicists happy, because it is much easier to calculate things when the forces are not very strong. The theory of radiation and charged particles, called Quantum Electrodynamics (or QED for short), is probably the best understood and the most successful theory in all of physics. Some of its predictions have been verified experimentally to twelve decimal places.
Another important property of α is that it is dimensionless. It is formed as a ratio of three of the fundamental constants of Nature: the electric charge of the electron, e; the speed of light, c; and Planck's constant, which is usually written as h(bar), and which is necessary to describe quantum phenomena. Each of these 3 constants has dimensions, that is, it changes when you change your system of units. For example, the speed of light is very close to 3x108 meters per second, but if we want to know it in miles an hour, the answer is a different number, 6.7x108. However, when we form alpha by taking the ratio α=e2/h(bar)c, all the dimensions cancel out. The result is a dimensionless number, whose value is 0.007297351 no matter what system of units we use.
The fact that α is dimensionless has led some physicists to speculate that its value has some fundamental significance. It describes a fundamental property of the universe, and the question is whether its value is just an accident of how the universe happened to be put together, or whether its value is determined by some deep principle that we have yet to discover.
| A note on calculating alpha If you look up these constants in a textbook, and form the indicated ratio, you may or may not get the advertised number. This is because in some systems of units, one must additionally divide by 4p and in others by a constant denoted by 4pe0. This does not mean that a depends on the system of units. Rather, it means that in different systems of units one must define the same physical constant, α, in different ways. Here is a rule that will give you α using the constants in a randomly chosen physics text: find the expression for Coulomb's law, and use this to give you the magnitude F of the force between 2 electrons separated by a distance R. The expression will have the form F = ke2/R2 What k is will depend on the system of units the book is using. But whatever it is, the way to extract the fine structure constant from the numbers in the book is to calculate α= ke2/h(bar)c Another tricky point to note is that many books define something they call Planck's constant and denote it by h rather than h(bar). The relation between these two is that h(bar) = h/2p. |
Links
Physical Review Focus
Physics News Update
PhysLink
UC Riverside