Dressed to Impress: Attraction Between Electrons
October 13, 2016
Like charges traditionally repel, but researchers have refined and confirmed a way to make two nearby electrons attract one another! How? It’s all about “dressing” them.
Review of Coulomb's Law — Force Between Charges
Charles Augustin de Coulomb first published the relationship between two charged particles in 1784. He found that the direction of the force between the particles depends on the charge type. If the two particles have the same charge, the force is repulsive along the line between the charges—they push each other away. If the two interacting charges have opposite signs, then there is an attractive force between the charges, pulling the charges toward each other.
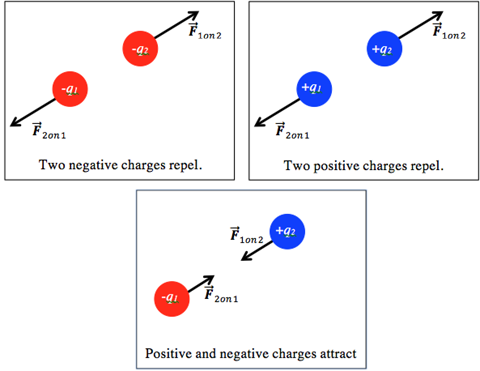
The strength of the force between the charges depends on two factors: the magnitude or amount of each charge and the distance between the charges. If the amount of charge increases, the force gets stronger. If the distance between charges increases, the amount of force decreases. Coulomb found the relationship between the size of the force and the variables it depends on to be:
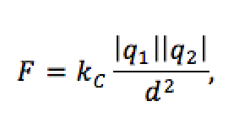
where kC is a constant called Coulomb’s constant, and d is the distance between the center of the charges squared, and |q1| and |q2| are the magnitudes (sizes) of the charges involved.
Fields and Potentials
If the force acting on a charged particle causes it to move, then energy has been imparted on the particle, because movement involves energy. The energy of motion is called kinetic energy.
Now imagine we just have one particle and we perform a “what if” scenario: What if a second charged particle comes near it? We know it would feel a force, but how much? To better frame this problem, scientists came up with the notion of an electric field that permeates space around a charge and provides an answer for this “what if” scenario. The electric field is just the force (from Coulomb’s law) divided by the second charge, taken to be a test charge of one unit of charge. In other words the electric field produced by a single charge is:
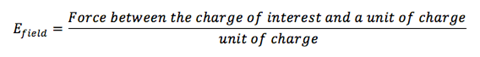
(It’s a force field!) If you want to know the force on any charge brought close by, all you need to do is multiply the electric field by the charge you are bring by. The way it is written here doesn’t let you know the direction of the force, but it is easy to figure out.
Continuing the “what if” scenario: Say a second charge, charge 2, is put it in the electric field created by charge 1, and just held in the field. In this case charge 2 has the potential to move, if it is let go—it has potential energy. We can describe this potential energy charge 2 has as its amount of charge multiplied by the electric field it is in, divided by how far away it is from charge 1.
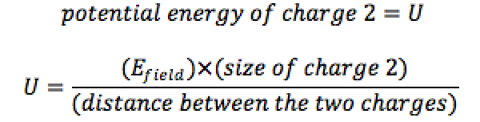
What’s nice about this is that potential energies lend themselves well to being depicted visually—scientists like to draw potential energy diagrams that look like hills and valleys.
Think of a ball let go at the rim of a valley; it will roll down to the bottom, because there is an attractive force acting on the ball pulling it toward the valley. In the valley example, the attractive force is gravity. At the bottom the ball has reached a lower potential energy state, and it is stable—meaning it is unlikely to roll up on its own. When a particle is near a potential energy “valley,” it is said to be near an attractive potential.
Now consider a hill. If you put a ball on the side of a hill it is very unlikely to roll upward, rather it will roll down the hill, away from the high point. It’s as if a repulsive force acts on the ball when it is near the hilltop. The lower-energy state is away from the hilltop. When a particle is near a potential energy “hill,” it is said to be near a repulsive potential.

The ball near a valley will roll in, being attracted to the low point. The ball on the hill will roll away, being repelled from the high point.
Bending the Rules
So how do scientists get two electrons to attract one another? The researchers used a system of electrons moving along a line on a nanotube. If the electrons are next to each other in the line, they usually repel, but when another set of electric charges is brought near, from above, it changes the potentials, turning a pure “potential energy hill” into a potential energy hill with a potential energy valley on one side. The pure repulsion between electrons turns to one in which the electrons are attracted.
Left diagram shows a repulsive potential energy felt by a nearby electron, and the right diagram shows how the potential energy is altered, becoming attractive, when a polarizer (charge that can move between two vertical positions) is nearby.
Image Credit: Avishai Benyamini and the Graphics Unit in Weizmann Institute.
Bringing in the charge from above (also on a nanotube) alters the potential energy hill, a process often referred to as “dressing.” The charge brought in from above is called the polarizer. The electron can move easily from the bottom to top position in the purple segment. As the polarizer is brought in from above, the “purple” electron is pushed to the top state by the green electron. The polarizing (purple) electron has its own potential energy hill associated with it., and combining the two potential energies leads to an effective potential energy around the lower green electron, which is the “dressed” potential energy. The valley that appears in the lower electron’s potential energy only arises due to the polarizer electron above it moving to its upper state.
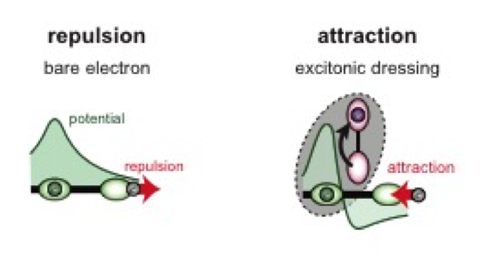
The researchers found that they can also attract electrons in two parallel chains by having the polarizing electron brought down from above, between the two chains as shown in the illustration below. As the purple electron gets pushed upward by the two nearby green electrons, the green electrons across from each other attract. The attraction is depicted by white lines between the green electrons.
Two parallel chains of electrons (green) are attracted when polarizing electrons (purple) are between the two chains from above.
Image Credit: Avishai Benyamini and the Graphics Unit in Weizmann Institute.
Future Research
Electron attraction is known to occur in superconductivity. During superconductivity, which only occurs at temperatures well below 0˚C, two electrons pair up. The paired electrons can be multiple lattice sites away (rather than right next to each other). This research may lead future researchers to novel materials that have properties such as superconductivity, but at much higher temperatures. The researchers note in their article1, that “the benefit of repulsion-driven attraction is that the pairing energy of the attraction increases linearly with decreasing device dimensions.” This means that the energies possible will be significantly larger than what they already observed.
References and Resources
1. A. Hamo, et al., Electron attraction mediated by Coulomb Repulsion, Nature 535, 395–400 (21 July 2016) doi:10.1038/nature18639
http://www.nature.com/nature/journal/v535/n7612/abs/nature18639.html
2. E. Conover, Electrons Have Potential for Mutual Attraction, Science News, 20 July 2016 https://www.sciencenews.org/article/electrons-have-potential-mutual-attraction
—H.M. Doss