Element 115 and the Island of Stability
September 24, 2013
We may have a new element to add to the periodic table! Element 115, temporarily called ununpentium (Latin for one-one-five), famously depicted in sci-fi flicks and video games, was indirectly observed in late 2012 and reported in the journal Physical Review Letters this month.1
Periodic Overview
The periodic table arranges elements in order by the number of protons residing in an element’s nucleus, the core of an atom. The table also organizes elements based on their chemical properties and the way electrons orbit their nuclei. Neutrons and protons reside in the nucleus. The protons are positively charged and repel each other, with the repulsion increasing as the protons get closer. However another force — the strong nuclear force — is stronger than this repulsion of charge, and holds the nucleus together.
Neutrons don’t have an overall charge, but they have some magnetic properties. Neutrons act as a kind of buffer between protons, which is why hydrogen atoms (which have one proton in the nucleus) have the only nuclei that can exist without any neutrons. Usually, the more protons you have, the more neutrons you need. In general, the elements two through twenty have about the same number of neutrons as protons, and then the number of neutrons become greater than the number of protons.
A given element doesn’t always have the same number of neutrons in it. The number of protons plus neutrons defines the atomic mass, and which isotope of the element you have. Isotopes of an element all have the same number of protons but vary in the number of neutrons. When writing an isotope, the overall number of protons + neutrons (nucleons) in the nucleus is listed. For example, uranium-238 is the element defined to have 92 protons and 146 neutrons (248 nucleons – 92 protons =146 neutrons). Uranium-235, which is used in nuclear reactors, has 92 protons and 143 neutrons.
Elements usually have the same number of electrons orbiting the nucleus as the number of protons. Electrons are much smaller than protons, and contribute negligibly to the atom’s overall mass. They have a negative charge with the same magnitude as a proton’s positive charge, so the overall charge of an atom with equal amounts of protons and electrons is zero or neutral. If an atom has any extra electrons or lacks any electrons then it is called an ion, and it has an overall charge.
On Earth we know of just 98 elements that occur naturally, some of which are very unstable and found in only extremely small amounts. If you look at the periodic table, the naturally occurring elements begin with Hydrogen (1) and end with Californium (98).
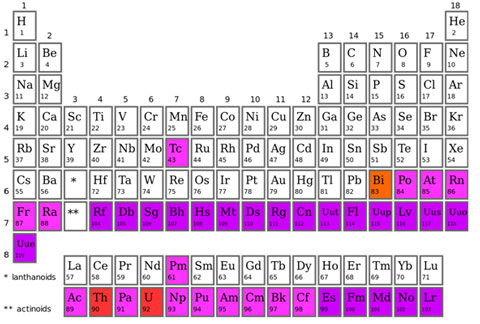
Periodic Table. White indicates naturally occurring stable elements, red are naturally occurring radioactive elements, pink are naturally occurring elements that are found in trace amounts, and purple are elements that only exist on Earth due to creation by humans.
Image Credit: Incnis Mrsi
The Island of Stability: From Sci-Fi to Reality
Now we turn to the newest candidate for induction to the periodic table: element 115. Element 115 pervades science fiction, video games, and some conspiracy theories3 because scientists theorize that some isotopes of this element may exist on the Island of Stability.
The Island of Stability follows from theoretical predictions that some heavy elements with a total number of nucleons around 300 may hang around for a while before radioactively decaying into other types of nuclei, unlike most other elements with nucleons totaling slightly under 300. What does “a while” mean? Minutes to millions of years.4
Experiments conducted by a Swedish research team published in 2013,1 along with those reported in 2004 by Russian scientists,2 show that element 115 was created, with a total of 287, 288, and 289 nucleons, and it has a half-life between 140 and 190 milliseconds.5 The half-life is the amount of time for half of the material to decay into another element. Element 115 is a stone’s throw from the coast of the Island of Stability.
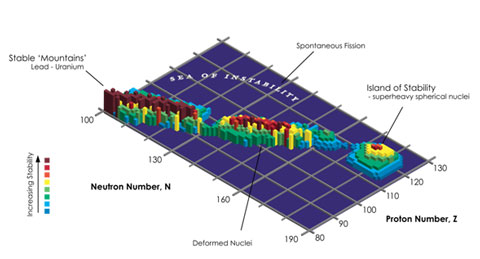
A diagram showing stable nuclei that would live on the theoretically predicted Island of Stability.
Image Credit: InvaderXan via Wikimedia Commons
Between the experimental results published in August 20131 and those from 2004,2 there should be enough evidence to forever place element 115 on the periodic table, with a new, permanent name. Everyone is just waiting for an international review panel to go over the experiments and make sure there is enough evidence.
Creating Element 115
To make element 115, scientists have to smash very high-speed calcium ions, which have 20 protons, into a target with americium atoms, which have 95 protons.1 If everything works just right, then there is a rare occurrence of a calcium ion fusing with an americium atom to create an atom with 115 protons that can exist for a few hundred milliseconds.
The calcium ions need to be going pretty fast, so the scientists requested time at the Universal Linear Accelerator in Darmstadt, Germany. They were lucky enough to get 8 of the 12 weeks they requested, but then found out they only had 3 weeks to do their experiment!
For three weeks, the scientists conducted the experiment non-stop, along with support from the technical staff at the accelerator facility. They created a pulsed beam of calcium ions. The beam of calcium ions was turned on and off; when a possible element 115 event was observed, the off-time was extended to continue measurements in a reasonably calm environment.
When the beam was on, there were about six trillion, energetic calcium-48 ions per second colliding into the americium target. The scientists made the americium target by electroplating diamericium trioxide (243Am2O3) onto a titanium foil, which faced the beam of calcium ions. The 6 trillion calcium ions per second collided into the americium target with energies between 242.1 to 245.0 MeV (million electronvolts), which corresponds to speeds of about 10% the speed of light!
Everything went well the first time the system was turned on (which is unusual in big experiments) and the scientists started seeing results on the first day of running the experiment. The signature of element 115, (what the scientists measure), is a series of radioactive alpha decays. Each time this occurs, the nucleus splits (decays) and emits an alpha particle, which is the same as a helium-4 nucleus.
After the alpha decay, the new nuclei (daughter nuclei) are usually in an excited energy level, meaning that it is not in its lowest energy state. The excited states decay into lower energy levels by emitting electromagnetic radiation in the gamma ray range, or following a process known as internal conversion, they emit X-rays.
Internal conversion occurs when an excited nucleus interacts with one of the inner electrons, causing the electron to eject out of the atom. The number of nucleons remains unchanged, and a higher level electron fills the vacancy left in the inner electron shell, emitting an X-ray as it makes the transition.
The measurement of the gamma-rays provided the scientists with insights into the nuclear structure of the decaying nuclei. The X-ray energies emitted are characteristic for an element, allowing the scientists to use these like a fingerprint to identify the number of protons inside a superheavy nucleus.
To measure the alpha decays and the electromagnetic radiation (gamma-rays and X-rays) scientists used the TASISpec (TASCA Small Image mode Spectroscopy) and TASCA (Transactinide Separator and Chemistry Apparatus). TASCA is basically a gas filled chamber with a magnetic field to separate charged particles. The americium target is at the entrance window of TASCA.
When the six trillion calcium-48 ions collide with the target, many different things can happen: fusion to create element 115, knocking particles out of the target, deflection, and a lot of forward moving calcium ions. In all of these, the forward motion continues, and a huge stream of particles enter TASCA. Once inside TASCA, the particles begin to get separated out, leaving only about 100 to 120 particles per second at the end and into the TASISpec. Of these 100 to 120 particles per second, only one or two per day are determined to be element 115.

Left: The TransActinide Separator and Chemistry Apparatus (TASCA) at GSI Darmstadt is a highly efficient device for studying elements.
Right: The thin housing of aluminum covers the silicon detection system (green framed box) developed by researchers at Lund University, Sweden to detect alpha particles. The germanium detectors that detect photons (gamma rays and X-rays) face the windows of the housing unit, and are pulled back in the image to better see the silicon detection system.
Image Credits: Gaby Ottos/GSI (left) and Unverisity of Lund, Sweden (right).
TASISpec uses a very thin (0.5 mm) aluminum housing everywhere that has minimal obstruction of the gamma rays and X-rays. The housing windows (green box in picture above) covers the strips of silicon detectors that detect charged particles such alpha particles.
Surrounding the box that houses the silicon detectors are germanium detectors (silver metal objects pointing at window faces of the green box) that detect gamma-rays and X-rays (energetic photons). During the experiment, the germanium detectors are very close to the windows of the housing unit. The scientists also measure how close in time the detectors detect an alpha decay and a subsequent energetic photon emitted as a new nucleus emits energy to go to a lower energy state.
The Swedish group detected about 1.5 instances each day of element 115 and about 10 per week. Again, they detected signatures of element 115: a specific chain of alpha decays and some gamma and X-ray radiation.

Inside the 120-meter-long linear accelerator at GSI, which accelerated the calcium ions used to produced element 115.
Image Credit: Gaby Otto/GSI Helmholtzzentrum für Schwerionenforschung Gmbh
Future Research
The quest continues to make super heavy elements and reach the Island of Stability. For now, we have a tiny bit more insight into the nuclear structure of super heavy elements, and better equipment to observe the different types of radiation they emit as they decay. This will help scientists figure out what to keep and what to add to the different theoretical models of atomic nuclei, which Dr. Rudolph describes as, “The core of all matter around us.”
These basic science experiments help theorists better predict the location of the Island of Stability. For now, that island is still out of reach. One of the difficulties is the need for more neutrons in the created nuclei, which experimentalists have not been able to do, yet. Another is the lifetimes of the elements on the Island of Stability are not really predicable at this time, and for any practical application these elements might have, they would need to hang around for hundred years or more.
Until then, science fiction continues to propose fantastic ideas for these new elements that can produce gravitons, power up weapons and time machines, release antimatter, cause disease, create zombies, and even speed up and alter evolutionary processes!3
References and Resources
1. Rudolph, D., et al., “Spectroscopy of Element 115 Decay Chains,” Phys. Rev. Lett. 111, 112502 (2013)http://prl.aps.org/abstract/PRL/v111/i11/e112502
2. Yu. Ts. Oganessian et al., “Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291-x115” Phys. Rev. C 69, 021601 (R) (2004).
http://prc.aps.org/abstract/PRC/v69/i2/e021601
3. Materials science in science fiction
http://en.wikipedia.org/wiki/Materials_science_in_science_fiction
4. Island of Stability, Nova Science Now (2006)
http://www.pbs.org/wgbh/nova/physics/stability-elements.html
5. Facts and Figures, Lund University, Nuclear Physics
http://www.nuclear.lu.se/english/research/basic_nuclear_physics/nustar/element-115-faq/facts-and-figures
—Heide Doss