A New Method for Reducing Quantum Uncertainty
May 2, 2017
Using a creative and insightful approach, scientists from the Barcelona Institute of Science and Technology (ICFO) recently measured the spin of atoms more precisely than many scientists thought was possible. Their method sidesteps a quantum mechanical source of uncertainty that usually clouds such sensitive measurements. With this new method comes the promise of instruments that can measure magnetic fields, brain activity, and even gravitational waves more accurately than ever before.
The ICFO researcher team (L-R): Ferran Martin Ciurana, Lorena Bianchet, Dr. Rob Sewell, Prof. Morgan W. Mitchell. and Dr. Giorgio Colangelo.
Image Credit: ICFO.
Atomic Spin
Atoms, the building blocks of matter, can be described by their charge, mass, composition, and size. These terms make sense to most people because they mean the same thing in the everyday, macroscopic world as they do when you zoom way in to quantum realm of individual atoms. Atoms can also be described by their spin. Atomic spin is harder to visualize because it doesn’t have an exact equivalent in the macroscopic world.
Like a basketball spinning on a finger, the spin of an atom is a kind of angular momentum. However, the spin of an atom doesn’t actually describe how fast the atom is rotating—its meaning is specific to particles in the quantum realm. There are similarities though, and in some cases it is helpful to think about spin in this way.
Unlike the spin of a basketball, the spin of an atom is influenced by magnetic fields. A magnetic field will cause something called spin precession in the atom, in which the spin of the nucleus precesses around the direction of the magnetic field like a wobbling top or gyroscope. Many high precision instruments, such as MRI systems, gather information about the tissue or sample based on the spin precession rate of atomic nuclei.
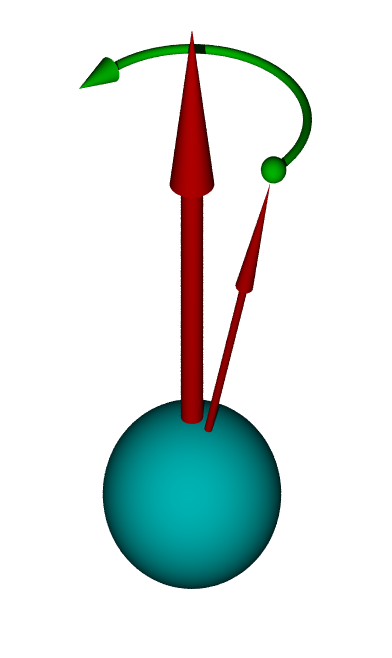
This image shows the precession of a negatively-charged particle in an external magnetic field. The large arrow indicates the external magnetic field and the small arrow indicates the spin angular momentum. Public domain.
The Heisenberg Uncertainty Principle
When “quantum mechanics” and “uncertainty” are mentioned in the same sentence, the Heisenberg uncertainty principle usually follows. This principle states that precisely measuring one property of an atom limits how precisely you can measure a complementary variable. The most common examples are position and momentum, as is illustrated in the following joke.
Have you ever heard the story about Heisenberg driving on the highway? He's speeding along, and he gets pulled over. The police officer says, "Do you have any idea how fast you were going?!" And Heisenberg says, "No. But I know exactly where I was!"
Unfortunately for drivers with no regard for speed limits, the Heisenberg uncertainty principle only holds on the quantum scale. The uncertainty relation is the result of atoms and other quantum objects behaving as both waves and particles. When a scientist measures position, for example, it’s impossible not to slightly disturb the momentum and introduce some uncertainty.
Spin precession can’t be directly measured, so spin-based instruments determine the precession rate by repeatedly measuring two variables, spin angle and amplitude. The common assumption is that the Heisenberg uncertainty principle applies to spin, meaning that the more precisely the spin angle is measured, the more uncertainty there is in the amplitude measurement—and vice versa. This sets the limit for the highest precision possible to something called the standard quantum limit. Current spin precession measuring techniques slightly disturb the spin variables as predicted and stay within this limit.
Quantum Uncertainty in Spin
In research recently published in the journal Nature, a team of scientists led by Giorgio Colangelo and Morgan W. Mitchell demonstrate that applying the Heisenberg uncertainty principle to spin puts an unnecessary limit on the precision with which spin precession can be measured.
Spin is actually described by three variables: spin amplitude and two spin angles. Imagine the atom is enclosed in a spherical shell. To describe a position on the shell, two angles are required—one describing the longitude and one describing the latitude. Although the Heisenberg uncertainty principle is the most well-known uncertainty relation, in reality there are different uncertainty relations for different systems. The uncertainty relation that applies to a system of three variables is different than the one that applies to two complementary variables like position and momentum.
As the team showed in this research, to determine the precession rate it’s only necessary to measure two of the three spin variables—the spin amplitude and the spin angle associated with longitude. By designing a measurement technique that pushes most of the uncertainty into the unnecessary angle (the spin latitude), the researchers we able to measure spin amplitude and the relevant spin angle to a precision much better than the standard quantum limit.
Reducing Quantum Uncertainty in Spin Precession
After showing that it is possible to reduce the quantum uncertainty in spin precession measurements, the researchers concentrated on doing it experimentally. This was no easy task. Some of the technologies they needed didn’t even exist yet, so the team had to create them. “It was a battle against the Dark Side of Quantum, but we won it!" says Colangelo.
The key was developing a non-destructive method. Like non-destructive testing methods that look for weak spots in metal infrastructure without disturbing the metal, non-destructive measurement techniques gather information without disturbing the things they are measuring. In this case, that means a measurement technique that doesn’t disturb the spin amplitude and spin longitude, but can disturb the spin latitude. In fact, it’s impossible to use a non-destructive method for this case that doesn’t disturb the spin latitude, because of the uncertainty relation.
Researchers Ferran Martin Ciurana and Dr. Giorgio Colangelo, working on the experimental setup.
Image Credit: ICFO.
What does this look like in practice? The researchers started with a cloud of more than a million atoms whose spins were aligned in the same direction. They cooled them down and then applied a magnetic field perpendicular to how the spins were aligned. This caused the spin to precess in a particular way.
To measure the precession, the researchers sent pulses of laser light through the atoms. Light interacts with magnetic fields in a well-defined way. By measuring the properties of the light after it passed through the cloud of atoms, the researchers could determine the magnetization, and therefore the spin in the relevant directions, of the atoms in the cloud.
The light does disturb the spin of the atoms as it passes through, adding uncertainty. However, the light is aligned with the initial spin of the atoms in a particular way so that it primarily adds uncertainty to spin's latitude angle.
“The nice thing about this interaction (from a quantum mechanical point of view) is that it doesn't perturb the spin orientation that is indirectly measured. In this way, you can measure it better and better, even better than its classical limits,” says Colangelo.
The researchers say that this method will enable significant increases in the sensitivity of state-of-the-art sensing and spectroscopy techniques. “Next we plan to study how this kind of spin tracking can be used in spin-based instruments to measure magnetic fields (for example from the heart and brain), and to measure time in atomic clocks,” says Mitchell. “We hope that others will look carefully at their own measurement techniques, to understand how sensitive the uncertainty principle allows them to be. There may still be surprises to be found.”
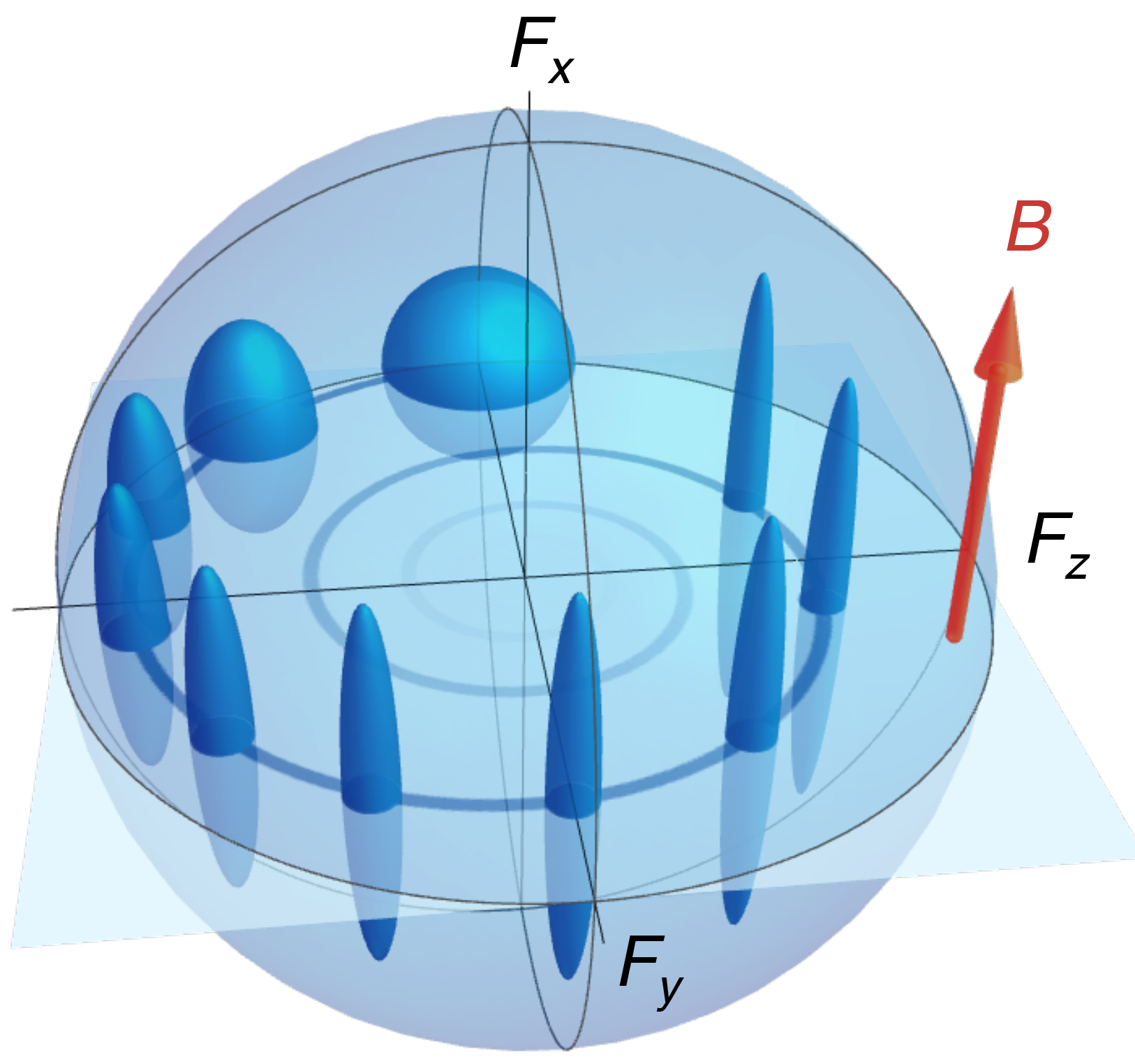
This image shows how the uncertainty evolves over time in this experiment. The dark blue shapes represent the uncertainty. Starting at the top (12:00), the dark sphere illustrates that the uncertainty is evenly distributed in all directions, Fx, Fy, and Fz. In the presence of the magnetic field indicated by the red arrow, B, the uncertainty evolves in the counterclockwise direction, being squeezed in the direction of Fx. This leaves the spin in the directions of Fy and Fz very certain.
References and Resources
G. Colangelo,et al., Simultaneous tracking of spin angle and amplitude beyond classical limits. Nature 543, 525-528 (23 March 2017) doi: 10.1038/nature21434
ICFO-The Institute of Photonic Sciences. Scientists evade the uncertainty principle. https://www.icfo.eu/newsroom/news/3469-scientists-evade-the-heisenberg-uncertainty-principle
—Kendra Redmond