Energetic Degenerates
If systems “seek” the lowest possible energy, why don't atomic electrons all cascade down into the ground state? Since the electronic properties of all atoms would be essentially the same, there'd be no need for the periodic table—we'd have no chemistry, and no life.
To the rescue comes the famous “Pauli exclusion principle,” which says that no two electrons may occupy the same quantum state. Imagine building up an atom by starting with a nucleus and adding electrons one by one. As each energy level is filled, it becomes unavailable for the next electron, which then must go in the next higher energy level, and so on.
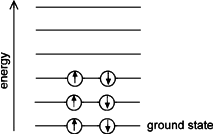
Fermi gas Energy Levels — Degenerate Fermi gas of particles with spin 1/2. Because of the Pauli exclusion principle, no two identical particles may occupy the same state. The particles are shown in each possible spin state, up and down. In this system, the particle energy is independent of its spin.
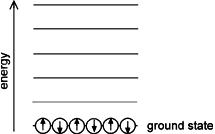
Bose gas Energy Levels —Bose gas at low temperature. All particles are free to occupy the lowest-energy state, also called the ground state. The particle energy is independent of its spin
The other family, Bose particles or bosons, have integer spin. They do not obey the exclusion principle and therefore can occupy the same quantum state at the same time, as shown in the diagram. Notice the difference in energy between the boson and fermion systems, which have the same number of particles.
Following groundbreaking low-temperature experiments with a gas of Bose atoms, which resulted in a form of matter called a Bose-Einstein Condensate (BEC) [See Matters of State], other researchers cooled fermionic atoms, looking for a degenerate Fermi gas, but this proved much more difficult. At a crucial stage in the cooling, the more energetic atoms are allowed to escape, and collisions among the remaining atoms ordinarily would redistribute their energy to bring the system back into thermal equilibrium at a lower temperature. In a degenerate Fermi gas, however, the low-energy states are already filled, so if two atoms collide, there are no lower-energy states available for the atoms to go to after the collision and they must stay in the original ones. Consequently, cold fermionic atoms cannot exchange energy by collisions, preventing the cooling of some of the atoms from lowering the temperature of the whole sample, as can be done with bosonic atoms.
Beyond electrons in atoms, the Pauli exclusion principle applies to any system of identical “fermions”—the family of particles with half-integer “spin,” the property that specifies intrinsic angular momentum. Fermions include the electron, proton, neutron, and other subatomic particles. A system of identical Fermions is called a “Fermi gas.” If the temperature is low enough, the Fermi gas is “degenerate,” which means the low-lying states are filled up to a well-defined maximum energy, as shown in the diagram. The degenerate Fermi gas provides a good model of the conduction electrons in a metal, even at room temperature. Also, in a white dwarf star, the electrons form a degenerate Fermi gas, and their pressure stabilizes the star against its gravity.
Research
To make a degenerate Fermi gas of atoms, physicist Deborah Jin, of the National Institute of Standards and Technology (NIST) in Boulder, Colorado, and her student Brian DeMarco prepared a sample of a Fermi gas of rubidium atoms. To get around the problem of the lack of collisions among very cold identical fermions, they put some of the atoms into a different quantum spin state by applying a radio-frequency electromagnetic field. Since the atoms in the different states were not identical, they could collide and trade energy. Allowing the highest-energy atoms to escape then could cool the whole sample as the remaining atoms collided and redistributed their energy. The sample eventually reached a temperature within a few millionths of a Kelvin of absolute zero, and its approach to the degenerate state is shown in the image with the three peaks.
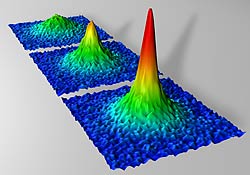
The emergence of the degenerate Fermi gas of atoms as the temperature is lowered (the image in the foreground has the lowest temperature). The sharpness of each peak indicates the relative number of atoms in the degenerate state. (image courtesy of NIST/JILA)

The group investigating the Fermionic condensate: group leader Deborah Jin, postdoc Markus Greiner, and grad student Cindy Regal (image courtesy of NIST/JILA, copyright Geoffrey Wheeler)
To determine if their cold sample was a degenerate Fermi gas, they turned off the trap and measured how the sample of gas spread out. If the sample were a degenerate Fermi gas of atoms, the sample would spread out rapidly, because atoms had populated relatively high-energy states, since the lower-energy states were already populated and thus unavailable. And that's what the images showed—a rapidly expanding cloud displaying the relatively high energy expected for a degenerate Fermi gas of atoms. More recent experiments have conclusively demonstrated such fermionic condensates.
With this experiment, and the corresponding discovery of the abovementioned BEC, experimenters within five years of each other, from 1995 to 2001, had found atomic systems predicted way back in the mid 1920s, in the great rush of theoretical work that grew out of the newly-developed quantum mechanics. It took experiment so long to catch up with theory because only around the Millennium did low-temperature technology become good enough to make possible experiments so close to absolute zero [see Laser Cooling and Trapping].
Beyond creating a degenerate Fermi gas, physicists want to investigate how the gas atoms interact, and many research groups are at work on this problem. A model of paired electrons explains the remarkable phenomenon of superconductivity [see Super Conductors], and paired helium atoms explain superfluidity, the flow without viscosity of helium at very low temperatures. To test this model, several groups are now actively searching for the superfluid state in an ultracold gas.
Links
JILA
PhysicsWeb
Closeup from image showing the emergence of the degenerate Fermi gas of atoms as the temperature is lowered. (image courtesy of NIST/JILA)