Graphene
A Substance Worth Writing About
By H. M. Doss
And the 2010 Nobel Prize goes to the André Geim and Konstantin Novoselov for graphene! Wait, isn’t that what’s in our pencils? Well, yes and no.
What Is Graphene?

Figure 1: Graphene
What is in our pencils is graphite usually mixed with some clay and sometimes some wax and/or charcoal. Graphite is a common structure of carbon atoms that consists of multiple layers of carbon atoms arranged in a honeycomb lattice. Graphene is a single atomic layer of this honeycomb lattice, which is composed of carbon atoms that form hexagonal rings. Graphene is considered two-dimensional because it is one atom thick. Before 2004 most scientists didn’t believe you could have a stable two-dimensional crystal, but in 2004 André Geim and Konstantin Novoselov discovered a way to isolate a single layer of these carbon atoms, and they did it with adhesive tape! By taking a small piece of graphite and rubbing it between two pieces of adhesive tape, and then pulling apart the pieces of tape, Geim and Novoselov were able to reduce the number of layers of graphene. They continued to gently rub the graphite between pieces of adhesive tape until they achieved a single atomic layer – or graphene! Watch the video. Geim, Novoselov and other scientists were then able to characterize the special properties of graphene.
Carbon Forms
The carbon atom is very versatile. It is the sixth most abundant element in the universe1 and the basis of all life we know. Carbon has four valence electrons, which allows it to bond to other carbon atoms in various ways. Carbon compounds form graphite, graphene, diamonds, and fullerenes, creating the strongest materials known to humans. Graphite, graphene, and diamond are just special names given to different structures of carbon atoms bonded with each other, or allotropes of carbon. These carbon molecules can become very large and only consist of carbon atoms that are arranged in a special way. Diamonds are large molecules of carbon atoms, but the arrangement of the carbon atoms is not in the same honeycomb pattern as graphene. Instead the carbon atoms join in tetrahedral units that then combine in a variation of a face centered cubic structure. An image of this structure is shown below.

Figure 2: Carbon allotropes - diamond and graphite
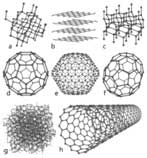
Figure 3:
a) Diamond
b) Graphite
c) Lonsdaleite
d) C60 (Buckminsterfullerene)
e) C540 (Fullerene)
f) C70 (Fullerene)
g) Amorphous carbon
h) single-walled carbon nanotube
Graphene is the strongest material known. But just how strong is it? To get an idea of how strong, James Hone, a Columbia University professor researching the material, described it this way, “Our research establishes graphene as the strongest material ever measured, some 200 times stronger than structural steel. It would take an elephant, balanced on a pencil, to break through a sheet of graphene the thickness of Saran Wrap.”2
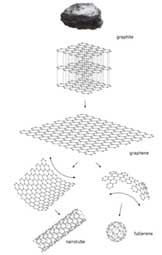
Figure 4: Graphite being made into graphene, which is then used to make a nanotube and fullerene. ©Airi Iliste/The Royal Swedish Academy of Sciences
Interestingly, graphene can also be stretched by a quarter of its length.3 This is due to the way the carbon atoms bond to each other. This property makes graphene useful as a sensitive resonator. Graphene is thin, light, a very good electric conductor, a good thermal conductor, transparent, and is highly impermeable, not even allowing helium to pass through. These amazing honeycomb lattice sheets of carbon can be wrapped up to create fullerenes that are shaped like a soccer ball, rolled to create nanotubes, or layered to create graphite. The layers of graphite are easily taken apart, which makes for a great substance for writing. Everytime you put your pencil to paper, layers of graphite come off the pencil and stick to the paper, making it useful for writing and drawing.
Nobel Prize Work on Graphene
André Geim and Konstantin Novoselov won the 2010 Nobel Prize in Physics for experiments they conducted with graphene that show it has exceptional properties originating from quantum physics. They were the first to isolate graphene - the thinnest, strongest material on Earth - and they isolated it with adhesive tape!
Geim’s experiments showed that the electrons in graphene behave in a special way. Graphene’s electrons behave in a relativistic way, although they move much slower than the speed of light in a vacuum.4 Graphene’s energy band characteristics leave it somewhere between a metal and a semiconductor.
Then Geim, Novoselov, and other scientists, began characterizing the properties of this material and found some extraordinary things. Graphene is the strongest two-dimensional substance and it can be stretched. It is an extremely good electrical and thermal conductor. It is almost impermeable, and it is almost completely transparent. There are many possible applications for this material that scientists are currently looking into developing.
Development
Scientists and engineers are trying to make high frequency transistors, surface coatings, electron emitters, and sensitive chemical detectors. Many predict that graphene transistors could contribute to the design of smaller and faster computers. Graphene is believed to be a superior material for creating touch screens, solar cells, electronics, single-molecule gas sensors, and sequencing DNA. By mixing graphene with other substances, such as plastics, scientists believe that they can make stronger, electrically conductive, heat resistant materials, which could be used to create strong, lightweight materials that could potentially improve aviation, automobiles, and satellites.
In 2008 the smallest (one nanometer) transistor known was created out of graphene, and a flash-like memory was created.3 A functional radio frequency transistor that uses graphene has already been created by GM and the Boeing owned laboratory, HRL.5
Some research published in November 2010 focuses on ways to introduce other atoms into graphene’s lattice to affect its energy spectrum. This would increase the scientists’ and engineers’ abilities to control the electronic characteristics.6
Graphene is being used to replace capacitors used for energy storage. Already nanotubes are being used as wearable electronics that utilize textiles coated with nanotubes. Electronic textiles are durable. They can be stretched (due to graphene’s elastic properties) and laundered without damaging the electronic properties of the material. Someday soon you may be recharging your cell phone with your clothes!7
References and Links
1. Math and Science Activity Center: The Carbon Atom
http://www.edinformatics.com/math_science/c_atom.htm
2. Columbia News, July 21, 2010,
http://www.columbia.edu/cu/news/08/07/graphene.html
3. Carmody, T. Why Graphene Won Scientists the Nobel Prize, Wired October 5, 2010.
http://www.wired.com/gadgetlab/2010/10/graphene/
4. Castro Neto, Antonio H., The carbon new age, Materials Today, 13, 3 pp12-17 March 2010
5. Mosher, D., Nobel Worthy: Best Graphene Close-ups, Wired October 5, 2010.
http://www.wired.com/wiredscience/2010/10/graphene-gallery/?pid=332
6. Mitra, S., Physics, spotlighting exceptional research, November 2010,
http://physics.aps.org/synopsis-for/10.1103/PhysRevLett.105.196102
7. Ganapti, P, To Charge Your iPod Plug into Your Jeans, Wired January 21, 2010.
http://www.wired.com/gadgetlab/2010/01/e-textile-charge/
8. Geim, A and Kim, P Carbon Wonderland Graphene, a newly isolated form of carbon, provides a rich lode of novel fundamental physics and practical applications, Scientific American April 2008,
http://www.scientificamerican.com/article.cfm?id=carbon-wonderland&page=6
9. http://nobelprize.org/nobel_prizes/physics/laureates/2010/