Super Efficient LEDs
More than 100% efficient, these Light Emitting Diodes (LEDs) put out more light energy than the electrical energy that they use!1 Remember that you can't get something from nothing, and energy must be conserved. So how does this Physical Optics and Electronics Group at MIT create such a super efficient LED?
Common light sources
It's long been known that LEDs can be far more efficient at producing light energy than regular incandescent bulbs, halogen lamp, and compact fluorescent bulbs.
 (a) |
 (b) |
 (c) |

(d) |
| Figure 1 (a) Incandescent bulb. Image credit: Wikimedia Commons (b) halogen lamp Image credit: Wikimedia Commons. (c) Compact Fluorescent Bulb, CFL bulb. Image credit: Wikimedia Commons. (d) LED bulb Image credit: Wikimedia Commons. | |||
According to the information listed at the US Environmental Protection Agency's website, incandescent bulbs only convert about 10% of the electrical energy input into visible light energy and about 90% is converted to infrared radiation (or thermal radiation). Compact fluorescent bulbs transform about 20% of the electrical input energy into visible light energy, and the remainder as infrared radiation. LED's come in a range of efficiencies, but are required to convert at least 25% of the energy input into visible light energy.
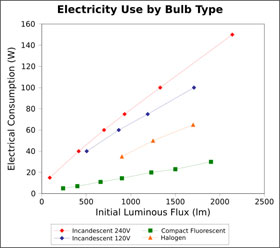 Figure 2. Graph showing energy input vs. light output for different bulbs.
Figure 2. Graph showing energy input vs. light output for different bulbs. Image credit: Wikimedia Commons
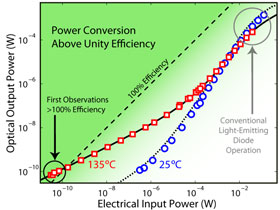
Figure 3. Graph showing the optical output power in watts versus the electrical input power in watts. The dotted line shows where 100% efficiency occurs. This is where the electrical input is equal to the optical output. Above the dotted line is where efficiencies of more than 100% occur. Image Credit: Parthiban Santhanam and Rajeev J. Ram, Physical Optics and Electronics Group at MIT.
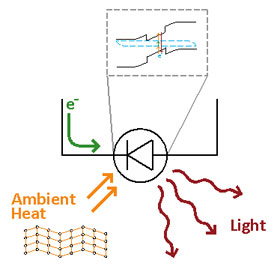
Figure 4. Color coded diagram showing electrical energy input (green), heat energy transferred to the LED from jiggling atoms in the lattice (orange) and light energy being emitted (red). The insert above the LED diagram shows the electron in the diode going from a lower to a higher energy level by absorbing the input energies, and emitting light energy as the electron returns to its lower energy level. Image Credit: Parthiban Santhanam & Rajeev J. Ram, Physical Optics and Electronics Group at MIT.
LED's aren't just created to produce visible light. Some operate in the ultraviolet range (with frequencies greater than blue and violet light), while others operate in the infrared range (with frequencies less than those of visible red light). They are efficient light sources, meaning that the amount of energy output that is deemed useful is a percentage of the energy they require to function. Usually the efficiency of an LED is well below 100%, no matter what type of energy it is emitting.
The LED created by the Physical Optics and Electronics Group at MIT doesn't emit visible light. It emits infrared radiation with a wavelength of 2.5 micrometers (0.0000025 meters) and a frequency of 120 terahertz (120000000000000 Hz). This is in what is commonly called the near infrared frequency range which spans 0.8 micrometers to 2.5 micrometers. It can't be used as a light source to read a book, but it might be used for other applications. The scientific observations are astounding. This LED operates by having a small amount of input electrical energy and emits more than twice that energy in infrared light! That means it is more than 200% efficient!
The graph in Figure 3 shows how the optical output power in watts is related to the electrical input power in watts. If you get the same power out as you put in, the LED is said to be 100% efficient. The dotted line shows where 100% efficiency occurs. Above the dotted line the LED has more than 100% efficiency.
Are you worried about energy conservation?
A fundamental law of physics is that energy cannot be created nor can it be destroyed. Energy can only be transferred from one object to another and/or transformed from one type of energy to another. For this LED, electrical energy is being transformed to near infrared energy, but more light energy is coming out than electrical energy is being put in! This does not mean that energy is not conserved. What is happening in this system is that the LED, a semiconductor diode, is not only having electrical energy transferred to it. It is also having heat energy transferred to it by transforming some of its thermal energy. Thermal energy is the jiggling around of the atoms that make this solid semiconductor, and the average jiggling around (average motion energy) is what we call the temperature of the object. The solid has a crystalline structure or repeating pattern that is called the lattice, and it has certain vibrations that are allowed. These vibrations make up some of the jiggling, and it is this vibrational lattice energy that is being transferred to some of the outer electrons in the material to move them up to a set of very closely spaced energy levels called a band. As the atoms and its parts jiggle less the LED cools down. It transforms this thermal energy and the electrical energy to near infrared radiation energy. When the lattice energy is transformed in this way, it is said that heat energy is transferred. This transformation of the lattice energy occurs when a small electric field interacts with the semiconducting material (LED) and energy is drawn from the jiggling atoms in the lattice to help move the electrons.
The researchers found a special set of conditions that allowed just the right situation for this to occur. One of the conditions they needed was to heat up the semiconducting material. Another condition was to have a weak electric field interacting with the material. When the LED achieved over 200% efficiency it was initially warmed to a temperature of 135˚C (275˚F).
The small amounts of energy transferred from the jiggling atoms in the lattice and from the electrical energy input into the system are absorbed by an atom in the LED, moving the atom's electron up to a band of closely spaced energy levels. When the electron goes back to its original energy level it emits all the energy as one big packet of electromagnetic radiation energy in the near infrared with a wavelength of 2.5 micrometers and a frequency of 120 terahertz.. No energy was created. No energy was destroyed. Energy was just transferred and transformed as required by the energy conservation law. A depiction of the energy levels and which energy is being transferred to the electron and emitted is shown in Figure 4.
Future research and applications
Although this incredibly efficient LED will not show up above your kitchen table anytime soon, it may be put to use. The overall amount of near infrared radiation is small, but if it can be distinguished above background radiation at this frequency, then it could be used as a tool in spectroscopy and/or optical communication devices (such as fiber optics devices), which currently utilize near infrared radiation in measuring, sensing, and transmitting devices. Current near infrared spectroscopic devices include applications for pharmaceutical products, diagnosing blood sugar and oxygen levels, forensics, neuroimaging, interfaces between neural activity and computers, neurology, neonatal research, quality control for food and agriculture needs, and many others.3
References, resources, and links
1. Santhanam, P., Gray Jr., D.J., Ram, R.J., Thermoelectrically Pumped Light-Emitting Diodes Operating above Unity Efficieincy, Physical Review Letters, 108, pp. 097403-1-5 (2012)
DOI: 10.1103/PhysRevLett.108.097403
2. US Environmental Protection Agency, Star Energy, Learn About LEDs
3. Near-infrared spectroscopy, Wikipedia (or google "near infrared radiation spectroscopy, applications")
4. Monroe, D. Optical Device is More than 100% Efficient, Physics, 27 Feb 2012
5. Wogan, T., LED converts heat into light, Physicsworld.com, 8 March 2011
6. Ackerman, E, 230% efficient LEDs seem to violate first law of thermodynamics, Dvice, 6 March 2012.
7. Green, D., Ultra-efficient LED puts out more power than is pumped in, Wired UK, 9 March 2012.
H.M. Doss