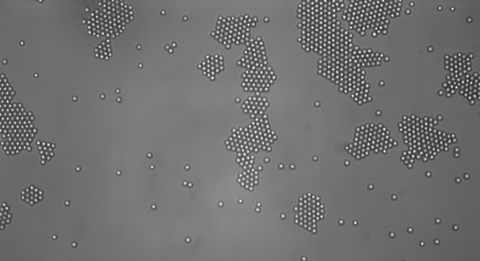
"Living" Crystal Colonies
A bacterium will group together with its neighbors to form a living colony, but what about non-living things? Researchers recently discovered crystals that form similar colonies when illuminated with a specific spotlight. But when this light goes off, the colony breaks apart!1 IT'S ALIVE! Well, not quite.
Self-Assembled Crystals
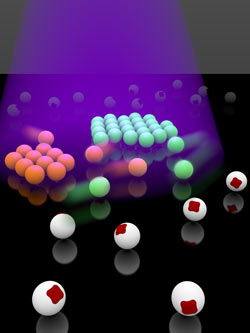 An illustration of the particles used in the experiment. The red square represents hematite and the white sphere represents the polymer that encapsulates the hematite to form this colloid.
An illustration of the particles used in the experiment. The red square represents hematite and the white sphere represents the polymer that encapsulates the hematite to form this colloid.
Image Credit: Kazem Edmond and Stefano Sacanna/NYU Center for Soft Matter Research
New York University scientists and a student from Brandeis University doing a summer research project2 recently uncovered this odd crystal behavior that mimics living creatures. These colloidal particles self-assembled into colonies under certain conditions, much like the formation of bacterial colonies, swarming insects, or flocking birds!
A colloidal particle is a particle that does not make a chemical bond with other particles. Instead, it remains suspended between particles of a different substance. Often the substance holding the colloidal particles is a liquid, but it could also be a gas or solid. Milk, for example, has colloidal particles in it. Its butterfat molecules are suspended within the liquid, and they can be seen under a microscope.
The colloidal particles used were made of two types of material: a polymer sphere made of 3-methacryloxyporpyl trimethoxysilane (TPM), that encapsulates most of an antiferromagnetic hematite cube.1 Under regular lighting or in the dark, these particles undergo the typically random motion caused by bombarding atoms and molecules in a fluid (gas or liquid).
Brownian motion describes this random motion of particles in time. Robert Brown conceived this idea while watching pollen particles in water under a microscope. He saw the pollen particles moving, but he couldn’t figure out why they were moving.
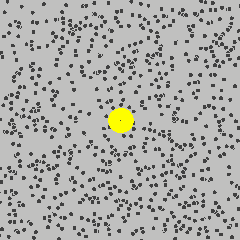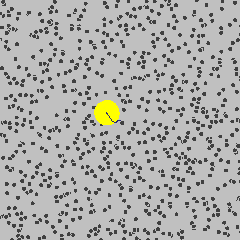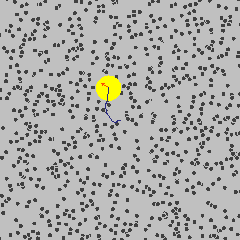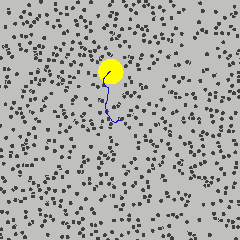
Simulation showing the motion of a dust particle (yellow) in a gas (for example air). The interaction between the gas particles and the dust particle cause the dust particle to move in its random Brownian motion.
Image Credit: Francisco Esquembre, Fu-Kwun and lookang
Brown then realized that it was the movement of the water particles and their interactions with the pollen particle that caused the motion of the pollen particle. The water molecules were moving around with regular thermal motion – the random motion of particles with temperatures above absolute zero.
For example, consider a dust particle in air. The air molecules are moving around randomly, as seen in the simulation to the left.3
The researchers observed Brownian motion when the colloidal particles were in a solution of hydrogen peroxide (H2O2) and illuminated with regular light. In this case, the colloidal particles and their surrounding solution are in equilibrium.1
When blue-violet light illuminated the system, the colloidal particles became self-propelled, forming colonies within 25 s after the blue-violet light came on. If they were suddenly attracted to each other, they would continue to colonize until all the particles were in the group, but that didn't happen.
Even after the colloidal particles grouped together into a crystalline structure, they still moved around, rotating and translating and changing colonies. Different colonies formed, and they rotated, translated, and collided. So the system is not an overall attractive system. If it were, they would all bunch together over a long period of time.
The average size of a colony was found to be 35 particles, and the typical time for a colony to change its size by 50% had a large range of about 100±75 seconds.1
When the blue-violet light was turned off, the particles “de-colonized” and exhibited Brownian motion again.
Watch the video below to see how light changes the system’s behavior.
Two Kinds of Attraction
To discover the impetus behind this colonization under blue-violet light, the researchers observed each material of the bi-material colloid separately. They attached just hematite cubes to a surface and placed polymer spheres in solution around it. When they shined the blue-violet light on the system, the spheres all moved toward the hematite cube, from all directions. Now the scientists wanted to find out why.
When hematite is submersed in the solution of H2O2 (hydrogen peroxide) and illuminated with the blue-violet light, it catalyzes the decomposition of hydrogen peroxide.1 This chemical reaction does two things: It changes the chemical composition of the fluid around the hematite and transfers heat energy to the surroundings, raising the local fluid temperature.
The researchers found that the difference in chemical composition affected the resultant motion of the polymer spheres more than the difference in temperature. Basically there was an energy transfer from the chemical reaction to the spheres that resulted in their motion and their attraction.
Scientists refer to this chemical composition difference as a concentration gradient, and the resultant change in motion of particles due to this is called diffusiophoresis. Temperature-based changes in motion are called thermophoresis.
It is the small attractive interaction from both of these phoreses that orders the aggregates into periodic (crystal) structures.
Future Research and Development
According to Jeremie Palacci, one of the researchers, this self-assembling crystal structure that can be controlled with a switch, could be used to study a variety of things. Palacci said that, “on the fundamental side, it sheds a new light on the emergence of collective effects in an assembly of active particles.”
Palacci gave examples such as the ability to reproduce the very cool collective effects we see in nature like flocks of birds or schools of fish. Scientists could reproduce these effects using a minimalistic system solely driven by physics.
Palacci also gave examples of technological applications. He suggested smart materials, where the particles could spontaneously self-organize under light into crystal structures, and materials whose property completely changes (mechanical, color...) under illumination. Palacci also pointed out that since this effect is reversible, you could create on-demand reconfigurable materials.
References and Resources
1. 1) Palacci, et al., Living Crystals of Light-Activated Colloidal Surfers, Science Express, 31 January 2013
http://www.sciencemag.org/content/early/2013/01/30/science.1230020.abstract
2. Devitt, J., NYU Physicists Shine a Light on Particle Assembly, NYU News, New York University, 31 January 2013
http://www.nyu.edu/about/news-publications/news/2013/01/31/nyu-physicists-shine-a-light-on-particle-assembly.html
3. Brownian motion, Wikipedia
http://en.wikipedia.org/wiki/Brownian_motion
—Heide Doss