Ultralight Lattices
Strong, springy, and ultralight, these lattices can sit atop a dandelion in seed without damaging it, and carry about 1000 times its weight without being damaged!

Figure 1 Microlattice on a dandelion.
Photo Credit: Dan Little, Copyright 2011 HRL Laboratories, LLC Malibu CA - All rights reserved.
What it means to be ultralight
To be within the category of an ultralight material, a density of about 10 milligrams per cubic centimeter is required. As an example consider an 8.5" by 11" sheet of copy paper (ultrawhite, 20 lb), which has a mass of about 4 g. Cut the paper in four equal pieces and now each quarter sheet has a mass of about 1 g. If the quarter sheet is cut into one hundred equally sized pieces, then each piece of paper has a mass of about 10 milligrams. That’s the mass, but density is also called for here. Imagine you could puff up the paper (like a cotton ball) so it would fill in a cubic centimeter (shown below). That would be ultralight.
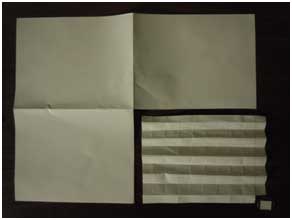 Figure 2 (a)A 1/400th a sheet of letter sized copy paper with a mass of about 10 mg. |
 (b)1/400th a sheet of paper on a penny, next to a cubic centimeter. |
Most ultralight materials are a puffed up gel (aerogel) with non-repeating patterns in their structure. Aerogels aren’t able to handle large forces acting on them without permanently being deformed. They also cannot absorb large amounts of energy and they are good insulators, but bad conductors. This newly developed ultralight material is highly structured and is special because it can handle large forces, absorb energy, and conduct electricity.
Why build a lattice?
Perhaps you have heard of this famous challenge (that has many variations): Build the tallest, strongest tower only using 40 straws and pins. You may cut the straws. It’s truly a challenge! Straws bend easily and collapse under heavy loads unless you have them just so. The lattice you create with the straws is very important in the final strength of the tower and its height.
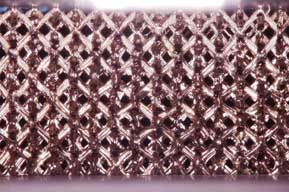
 Figure 3 |
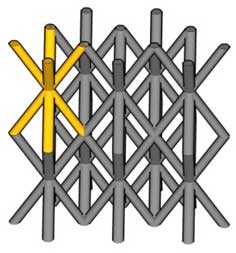 (b) Microlattice structure of newly developed ulatralight material. Image Credit: Copyright 2011 HRL Laboratories, LLC Malibu CA - All rights reserved. |
 (c) The Eiffel Tower which has a density similar to an aerogel.1 Image Credit Wikimedia Commons. |
The lattice structure of this new ultralight material was designed to do more than just be strong and light. It was also designed to be springy and absorb energy. It can be "scrunched" and then go back to its original shape, like a spring!
 (a) |
 (b) |
Figure 4 The microlattice goes back to its original shape after being squished. |
|
|
(c) YouTube video uploaded by ChemistryWorldUK |
|
Scientists call the amount an object is deformed compared to its original dimensions the strain. The microlatttice above can exceed strains of 50% and still go back to its original dimensions! Well almost – it reaches 98% of its original height.1 It has a super-elastic property. An elastic material possesses properties that push or pull the object back to its original dimensions (a restoring force). This remarkable structure does obtain some microscopic fractures that decrease its energy absorption during the first three times it is compressed, but then it maintains relatively constant energy absorption. It is these fractures or cracks that occur near junction points of the cell that cause it to lose 2% of its height after it is compressed. After the third compression, stable fractures are formed and the material has a relatively constant super-elastic behavior.
Constructing the microlattice
The metal beams in this microlattice structure are hollow, allowing for this structure to be ultralight. To create this structure the HRL research team first needed to decide on the repeating cell structure they wanted. They figured out the length of each beam and the angles involved. Then, they created a template using a polymer. The polymer starts off as a liquid that hardens when ultraviolet light shines on it. The researchers used a "patterned mask" which is similar to a stencil. When ultraviolet light shines through the open areas of the mask onto the liquid polymer, the areas of liquid polymer that are exposed to the ultraviolet light harden. Through this method the researchers created a three-dimensional array of repeating cells. The next step is to coat this polymer template with metal. The HRL team chose to coat the polymer with nickel phosphorous, using a process that allowed them to control the thickness of the metal coating. For example, they could coat the polymer structure with 100 nm (0.0000001 meters) of nickel phosphorous. (This is about 100 times thinner than a human hair!) Then the polymer template is removed by etching it out. They also noted that if they apply a thick layer of nickel phosphorous (1400 nm or 0.0000014 meters), then it crumples, not recovering, after a 50% strain is applied.
Future research and applications
Currently, ultralight materials are being researched for applications such as thermal insulations, absorption or damping of vibrational energy, sound energy, thermal energy, and battery electrodes. This new ultralight material holds new possibilities. It may still be used for all the current applications of an ultralight material, and perhaps other applications. It absorbs a greater amount of energy, is elastic so that the energy is recoverable, has higher thermal and electrical conductivity, and is highly structured. The fact that it is highly structured opens the doors to a whole new realm of ultralight materials, those that are highly structured and easily designed for specific purposes. Perhaps this metallic ultralight microlattice, which conducts electricity and can handle temperatures exceeding 400 ˚C, could be applied in aerospace structures such as satellites, space telescopes, and airplanes.
References, resources, and links
1. Schaedler, T.A., et al., Ultralight Metallic Microlattices, Science 334, 962 (2011)
2. Wilson, M., Building Ultralight Lattices, Phys. Today, 65 (1), 13 (2012)
3. Peralta, E., Cool Photo: Scientists Present ‘Lightest Material on Earth,’ NPRs News Blog, the two-way, 18 November 2011.
4. Physics Central Buzz Blog, World’s Lightest Material Unveiled, 18 Nov 2011
H.M. Doss