Water Tubes
About Neutron Diffraction
Physicists have created a new form of water, one that stays liquid at hundreds of degrees C below zero. This novel state of H2O occurs when water molecules are trapped inside a nanotube (see drawing), a recently-discovered form of carbon that is a close relative of the Buckyball (see the Physics in Action feature Tubular Peas).
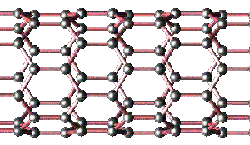
Side view of nanotube cylinder showing network of interlocked carbon atoms; the nanotube is a graphite sheet, rolled up into a cylinder (Copyright V. H. Crespi. Distributed under the Open Content License)
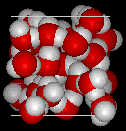
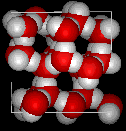
The molecular structure of liquid water, left, and ice, right. The fixed arrangement of hydrogen bonds between hydrogen and oxygen atoms opens up the structure of ice. (image courtesy of MathMol, New York University)
Water is a polar molecule, so the electrons are unevenly distributed, with an excess of negative charge on the oxygen atom and positive charge on the two hydrogens. The resulting electrostatic forces between neighboring water molecules form hydrogen bonds between the oxygen atom of one and a hydrogen of another (a total of four bonds per molecule), which produces the crystal structure of ice. The open atomic arrangement in ice (see drawings) is responsible for the decrease in the density of H2O upon freezing. In liquid water, the molecules also form hydrogen bonds, although they break and reform rapidly.
The structure of nanotube water is studied by neutron diffraction. You’ve no doubt seen the colored spectra produced when white light strikes a compact disk. This effect is an example of diffraction, which is a fundamental property of waves that can be used to investigate the nature of materials. Diffraction from an object becomes significant when the wavelength is comparable to the characteristic spacing—for the light scattering from the CD, when the wavelength of light is comparable to the distance between the grooves.
Visible light won’t work to investigate materials, since the wavelength of light is thousands of atoms long. To get down to typical atomic spacings, material scientists use x-rays. X-rays are scattered by the electrons in atoms, in proportion to the number of electrons. Although x-rays work well for investigating most crystals, they can’t be used to locate hydrogen atoms, because hydrogen has only one electron, and it scatters x-rays only weakly.
That’s where the neutrons come in. Neutrons, like other atomic and subatomic particles, have properties of both particles and waves. In fact, the relationship between momentum and wavelength—they are inversely proportional—is the same for both matter waves and for photons (the particles of light).
Since neutrons are uncharged, they are highly penetrating. Moreover, they scatter strongly from the atomic nuclei, and they clearly reveal the position of hydrogen atoms in the presence of heavier elements—just what is needed to investigate ice. Also, a related method, neutron inelastic scattering, shows how the energy is absorbed from the neutron beam, providing information about how the atoms are moving.

Argonne National Lab’s Intense Pulsed Neutron Source (IPNS) experimental area (photo courtesy of Argonne National Laboratory
A disadvantage of using neutrons is that it takes a big, expensive facility to produce them. Neutron scattering experiments originally were performed at nuclear reactors, but more recent facilities, such as Argonne National Lab’s Intense Pulsed Neutron Source (IPNS), produce neutrons from a pulsed beam of accelerated protons (see photo). The pulsed proton beam slams into dense metal targets (uranium at IPNS), setting off nuclear reactions that yield a hail of neutrons. The resulting pulsed neutron beam is then intensified by reflection from blocks of beryllium. The IPNS produced the beam of neutrons that probed nanotube water.
Research
Nanotubes, which are closely related to buckyballs, are cylinders of carbon, structured as if a sheet of hexagonal carbon were wrapped up into a cylinder. One end of the cylinder is closed with a half-buckeyball structure, as shown in the diagram. The diameter of the nanotube is about a nanometer.
Water molecules can fit inside a nanotube, but there is not a lot of room to spare. The hydrogen-oxygen bond length in water is about 10-10 meters, compared with the nanotube diameter of about 10-9 meters, so the fit is tight. Moreover, ordinary ice will not fit because of its open structure (see diagram in the previous section).
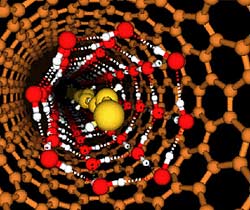
This image shows nanotube water inside a nanotube at low temperatures. In the drawing, the carbon atoms of the nanotube are brown; the oxygen and hydrogen atoms of the ice sleeve just inside the nanotube are red and white; the oxygen and hydrogen atoms of the liquid water inside the ice cylinder are gold. Notice that there is insufficient space for the open structure of ordinary ice. (image credit: Christian J. Burnham )
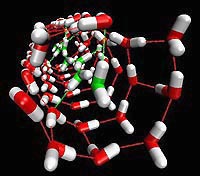
This image shows only the nanotube water, again at low temperatures. The red and white water molecules are the ice sleeve, and the green and white molecules are the liquid water inside. (image credit: Christian J. Burnham )
To study nanotube water, physicists marshalled several different experimental approaches and computer simulation as well. Neutron diffraction can be used to determine the structure of the water confined inside the nanotubes. Then neutron inelastic scattering revealed how energy passed from the neutrons to the nanotubes and water, particularly at low temperatures. This showed how the water molecules were moving and vibrating and also how the bonds among them formed and reformed. Finally, computer simulations of how the water molecules moved showed that the structure and the observed motion were consistent.
Here are the results. At low temperatures, the tight fit inside the nanotube forces the water molecules into an unusual structure. At eight kelvins (i.e., eight degrees above absolute zero), the water inside forms a thin sleeve, a cylinder of ice one molecule thick, as shown in the diagram, with liquid water inside—still liquid at -265° C! The two hydrogens of each interior molecule bind to oxygens of others, but the average number of bonds for these molecules is less than two, completely different from liquid water at room temperature. The molecules of low-temperature nanotube water are far more mobile than the molecules of bulk water at room temperature, and the bonds constantly break and reform. Contrast the diagrams of the nanotube ice sleeve and the liquid water inside with those of normal ice and water in the previous section.
The researchers plan to work with nanotubes with even smaller diameters, which may help them understand how membrane proteins transport water. Also, they plan to investigate what happens to the water inside as it is heated and cooled.
And for the field of neutron scattering as a whole, an important new facility—the Spallation Neutron Source at the Oak Ridge National Laboratory—is coming on line. It will be the most intense source of pulsed neutrons available and should become a center for the growth of neutron science.
Links
Argonne National Laboratory
New York University

The 750 KV preaccelerator of the Intense Pulsed Neutron Source (IPNS) (photo courtesy of Argonne National Laboratory)