Below Absolute Zero: Negative Temperatures Explained
Absolute zero, or 0 degrees Kelvin, is the temperature where all motion stops. It's the lowest limit on the temperature scale, but recent news articles have heralded a dip below that limit in a physics lab. Is absolute zero less absolute than we thought? Read on to find out.
Arctic Atoms

Researchers at the Max-Planck Institute for Quantum Optics and the Ludwig-Maximilians University Munich worked with a Bose Einstein Condensate of about 112,000 very cold potassium atoms (39K). A Bose Einstein condensate is made up of atoms that can occupy the same state.1
They put these atoms in an optical dipole trap.1 The dipole trap confines the atoms and keeps them together. They then used a tunable three-dimensional optical lattice that acts like a crystalline structure for the potassium atoms to reside in and creates an associated energy band structure.1
The optical lattice consists of a bunch of laser beams set to create a standing wave that acts like a lattice structure for the potassium atoms to be in. The potassium atoms in this lattice structure remain in their lowest internal energy state, but they have an associated band structure. The lattice confines the motional states of the atoms to this lowest energy band; however, this band consists of many states (momenta states) for each atom.
The researchers observed that the atoms could all be in their lowest energy band level and very near to the lower edge of that energy band level. Consequently, the system was extremely ordered (the amount of order is key to this) and, as you might expect, very cold. Keeping the atoms in this low-energy state led to a temperature of only a few billionths of a Kelvin.1 This situation resembles placing a bunch of balls into a small valley, where it's extremely unlikely for them to roll out.3
By very suddenly turning the valley of the optical lattice into a hill, the researchers essentially “flipped” the entire system around, causing the atoms to go into the upper edge of their lowest energy band. This is akin to turning a stable valley into a stable hill. This new “hill” is very stable, and the atoms would not roll off the hill to another energy state. In this situation the atoms are very ordered, cannot go to another energy level, and have a negative temperature value!
Below is an image of the momentum distribution at negative temperatures of the potassium atoms in the optical lattice. The momentum is proportional to the kinetic energy (motion energy) of the atoms, and the peaks show the maximum kinetic energy. The negative temperature states are very stable, and the atoms do not go to lower motional energy states.1
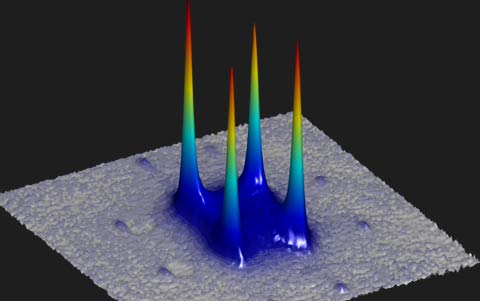
Momentum distribution when the atoms are in the upper levels of their lowest energy band. The peaks correspond to maximum kinetic energy (motion energy).
Image Credit: LMU/MPQ Munich
What is Temperature Anyway?
In an everyday sense, temperature describes an objects warmth or coolness. In science class, we are told that the temperature of an object is defined as the average kinetic energy (motion energy) of all the atoms that make up an object. The more the atoms that make up an object jiggle around (more motion energy), the warmer the object is.
It makes sense that if a warmer object is next to a cooler object, heat energy will transfer, from the warmer object to the cooler object. Think about those moving particles colliding with the cooler ones next to it (heat transfer by conduction). Similarly, the warmer particles that are in a higher energy state go to a lower one, radiating an electromagnetic wave in the infrared region and another atom absorbing that radiation (heat transfer by radiation).
Two objects of different temperatures eventually will reach a common temperature somewhere between the warmer object’s initial temperature and the cooler object’s initial temperature.
But there are other types of temperatures and other definitions of temperature. The one of importance for “below absolute zero” has to do with a definition used in thermodynamics and statistical mechanics. In thermodynamics, temperature is a macroscopic property, and the microstates of the system determine this and other properties.
For example, consider a system with 100 atoms and only two energy states. There is only one possible way to have 100 atoms all in their lowest energy state. If we take the logarithm of this one possible state, log(1), we get zero. Scientists call this the entropy of the system – a measure of the system’s disorder. Higher entropy means more disorder.
If just one of the 100 atoms in this system exists in the higher energy state then there are 100 ways to do this. In this case, the entropy is log(100) = 2. Recall that the researchers used about 112,000 potassium atoms.
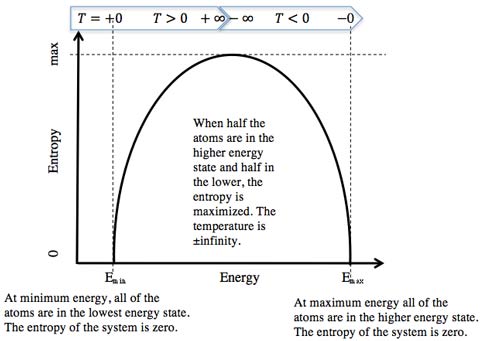
This means that entropy for this system is maximized when half of the atoms are in the higher energy state and half are in the lower energy state. If we reach the other extreme where all of the atoms are in the excited state, then there is only one way to do this and the entropy is again zero.
In statistical mechanics and thermodynamics, temperature is defined as follows:
1/temperature = change in entropy/change in energy
For our hypothetical system of 100 atoms with only two energy states, there is an upper and lower bound of energy, and the entropy of the system equals zero at these boundary limits. This definition of temperature can go from positive infinity to negative infinity. The temperature makes a discontinuous jump at the point where the entropy changes from an increasing value to a decreasing value.
The temperature for all the atoms in their ground state is zero. This is like a bunch of balls sitting in a valley.3
As energy is added to the system, some of the atoms go to the higher energy state, the entropy increases, and the temperature increases. This is like moving some balls out of the valley and onto the hill.
As more energy is added, more atoms go to a higher energy level. The temperature approaches positive infinity as the entropy nears its maximum, which occurs when half of the atoms exist in the upper state and half are in the lower state. In the hill/valley analogy, half of the balls would be in the valley and half would be on the hill.3
Still adding more energy, just as you reach the state of half the atoms being in the upper state and half in the lower state and pass it, the entropy of the system decreases. This results in the temperature discontinuously jumping from positive infinity to negative infinity (right at the halfway mark). This transition resembles the jump from having just less than to just more than half the balls on the hill and half in the valley.
As the system reaches its maximum energy state, the atoms all are in their upper energy state, the entropy goes to zero, and the negative temperatures increase, approaching zero. This is like all the balls being very stable on the hill, and not being able to fall off the hill.3
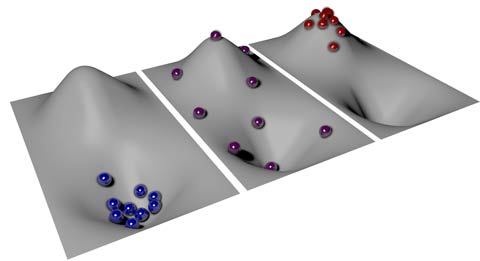
Image Credit: LMU/MPQ Munich
Does Negative Mean Colder?
It is important to note that the negative temperature region, with more of the atoms in the higher allowed energy state, is actually warmer than the positive temperature region. If this system were to be brought into contact with a system containing more atoms in a lower energy state (positive temperatures) heat would flow from the system with the negative temperatures to the system with the positive temperatures. So negative temperatures are warmer! And all this has to do with the how we define temperature.
One result from this experiment is that when the gas of potassium atoms (which happens to be a Bose Einstein Condensate) is experiencing negative temperatures, it is also experiencing negative pressure.1 If the pressure were not negative, then these highly attractive potassium atoms would collapse on each other.
This idea of an attractive system not collapsing is similar to the universe wanting to collapse due to the attractive nature of mass. However, the universe is expanding according to all our measurements and scientists have determined that something must be there to cause it not to collapse.
Scientists have called this something “dark energy,” because we can’t detect it. The researchers believe that perhaps the results of negative pressure can give insights into the ideas of dark energy, or a non-collapsing universe.
The researchers have also listed a number of research areas that the results of this experiment suggest. These include studies of: the Mott insulator transition, the parameters in the Hubbard model, and many-body systems.1
The Mott insulator transition is the transition between a material acting as an insulator or conductor of electricity. This transition usually occurs at low temperatures and is related to interactions between electrons. The Hubbard model is used to describe the transition between conducting and insulating systems.
References and Resources
1. Braun, S., et al. Negative Absolute Temperature for Motional Degrees of Freedom, Science (309) p. 52-55, 4 Jan 2013
http://www.sciencemag.org/content/339/6115/52
2. Chase, Scott, Below Absolute Zero – What Does Negative Temperature Mean?, Physics FAQ
http://math.ucr.edu/home/baez/physics/ParticleAndNuclear/neg_temperature.html
3. LMQ/MPQ research group, Negative Absolute Temperature, Frequently Asked Questions
http://www.quantum-munich.de/research/negative-absolute-temperature
—Heide Doss