Molecular Machinery Snags 2016 Nobel in Chemistry
November 22, 2016
The 2016 Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa “for the design and synthesis of molecular machines.” These are the world’s smallest machines—so far!
Machines
We often learn of simple machines in elementary school: the inclined plane, wheel and axle, lever, wedge, pulley, and screw. In each case the machine acts to change a force’s size and/or direction. These fundamental machines, combined with an energy input and devices like a motor, can make machines as simple as a wheelbarrow or as complex as cars, elevators, and cranes. The common electrical motor transforms the electrical input energy to mechanical energy. In general, a motor transforms an input energy to mechanical energy in order to move something, usually by rotation.
In 1959, Richard Feynmann proposed a challenge to fellow scientists: who could build the smallest machine? This year’s Nobel prize in Chemistry goes to just this category!
The First Steps—Mechanical Molecular Bonds
Bonds between atoms and molecules occur due to charges interacting with each other and forming a stable system—one that exists in a lower energy state than its component parts. Essentially, if it takes energy to pull the pieces apart, the combined system is a lower energy state in energy than its parts. For example, when two hydrogen atoms join together to form H2, the sharing of the electrons between the two atoms allows for a lower energy state than if each hydrogen atom were on its own. This sharing of electrons is called a covalent bond. Another type of bond, where one atom takes electrons away from another, is called an ionic bond. In this situation, both atoms end up in lower energy states, but each now has a net charge, either positive or negative. When an atom takes or gives an electron and is no longer neutral, it is called an ion. A positive ion is strongly attracted to a negative ion, due to the opposite charges, meaning the two take energy to pull apart, telling us that this is a lower energy state. An example of this type of bond is sodium chloride (NaCl), also known as table salt.
Since the 1950s, scientists have imagined binding molecules mechanically, linking two ring shaped molecules or putting a ring shaped molecule on a “dumbbell”—a linear molecule with ends larger than the diameter of the ring.
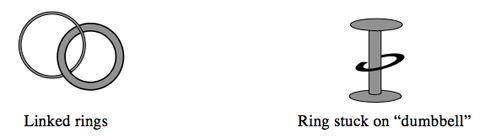
One of the first major milestones in the development of molecular machines was the creation of such linked molecules. This led not only to new types of molecules, but also a new type of bond–a mechanical molecular bond. Molecules bound as linked rings are called catenanes. Ringed molecules over dumbbell shaped molecules are called rotaxanes.
The Molecular Roll
Jean-Pierre Sauvage is responsible for creating a straightforward and efficient way of creating catenanes (linked ring molecules). In 1983 Sauvage and his team used a copper ion to basically pin down a half a ring, and then attach the other half. As the second half comes near, it too is attracted to the copper ion, but covalently bonds with the half ring present. After the two rings are linked, the copper ion is removed.
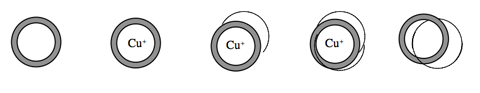
Sauvage's method for linking ring molecules: introduce copper ion to a ringed molecule, bring in half ringed molecule, bring in second half ring, remove copper ion.
Later, Sauvage’s group showed that keeping the copper ion in the middle and electrochemically altering it from Cu+ to Cu2+ could cause a controlled rotation of 180˚ of one of the rings. By going back to Cu+, they reversed the rotation. Being able to control a ring the size of a molecule to rotate half a turn forward and half a turn back might not seem like much, but it was a huge and essential start to building molecular machines.
The Molecular Shuttle
In 1991, Sir James Fraser Stoddart and his group found a way to efficiently create rotaxanes. He used ringed molecules called aromatics, which have special properties due to a closed ring of atoms. He took aromatic molecules that either needed an electron or wanted to give one away and utilized these to link an aromatic ring around a linear molecule with big ends (like a dumbbell). The aromatic molecule could than shuttle back and forth along the linear molecule.
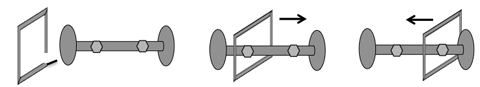
By 1994, Stoddard’s group was able to control the motion of the ring between two points by introducing specific molecules that either donated or accepted electrons along the linear molecule (shown as small hexagons in the diagram above). Altering the charges of the two site molecules would then move the aromatic ring along the length of the dumbbell structure.
More Machines & the Motor
The two groups collaborated and both found ways to alter the systems and control their motions using thermal inputs, light inputs, and chemical inputs. One such alteration led to a chain of rotaxane structures that could be thermally controlled; this resulted in contraction and relaxation of the molecules, similar a system of muscles. Molecular elevators were created, and molecular cantilevers.
By 2006, Stoddart’s group produced a motor using the rotaxane form, in which light triggered the ring to move up and down between sites a thousand times per second. As cool as this is, the limitation of the distance traveled has made it hard to find useful applications so far.
Common motors in big machines are typically composed of loops of wire that carry current and interact with a magnetic field. They transform electrical energy into mechanical energy to do things like turn a fan blade or rotate the tires on vehicles. This freedom to rotate continuously around in one direction makes them very useful.
The real breakthrough on molecular motors came later and involved the catenanes. The goal: to roll. But this requires that linked molecules rotate through a full 360˚, rather than the half circle and back that Sauvage’s design was capable of.
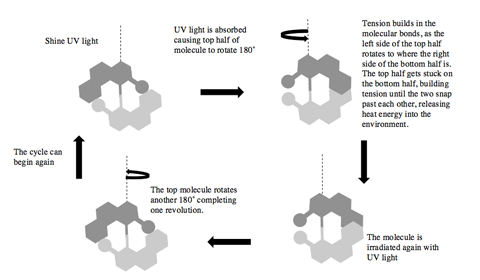
It was the third Nobel prize winner, Bernard Feringa, who made this breakthrough in 1999. As shown in the diagram below, Feringa created a molecule composed of two symmetrical halves. When irradiated by light, half of the molecule would rotate by 180˚, creating tension at the site where the two halves meet. The increased tension is finally overcome, allowing one molecule to slip past the other and releasing heat energy into the surroundings. Another pulse of light shining on the molecule then continues the rotation to complete the full turn, and again the connecting bond snaps and reassembles. After multiple refinements of this method, Feringa, in 2014, was able to get rotation rates of over 12 million cycles per second! And along the way, he built a four wheel drive molecular car that he could control!
Future Research
The future of molecular machines is wide open! Small machines continue. From shuttles, motors, tweezers, propellers, and switches, to gyroscopes and logic gates.
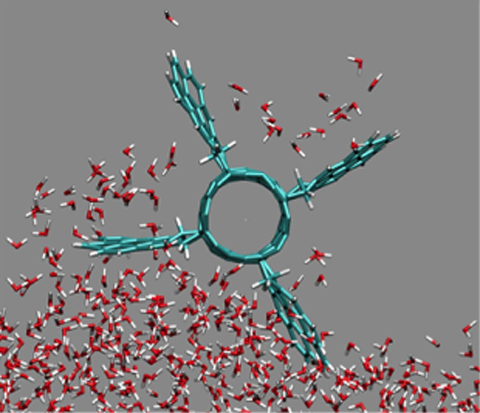
A molecular propeller uses hydrophobic molecules to push water molecules around.
Image Credit: Peter Král, Assistant Professor. Department of Chemistry M/C 111. University of Illinois at Chicago.
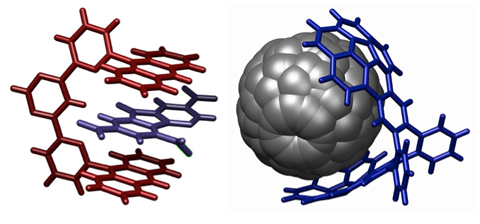
Left: One form of "molecular tweezer" (red) closes on its target molecule. Left: Another form of tweezer (blue) grasps a molecule of buckminsterfullerene—a carbon nanosphere.
Image Credit: English Wikipedia user M. Stone. CC BY-SA 3.0
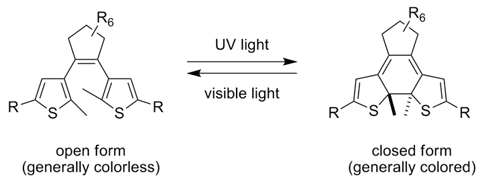
This molecule can switch from being colorless to colored depending on the wavelengths of light it's exposed to. This technology may have applications in powering the molecular machines of the future.
Image Credit: English Wikipedia user I'm with gerrit.
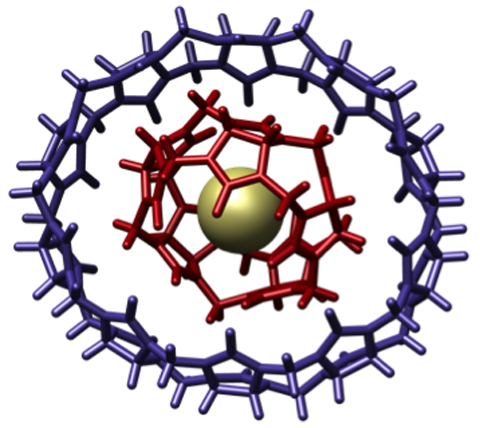
A "molecular gyroscope" comprised of a single chloride ion surrounded by carbon chains.
Image Credit: English Wikipedia user M. Stone. CC BY-SA 3.0
References and Resources
1. Nobel Prize Organization
https://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/
2. T. Kudernac, et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface, 479 (208-11) Nature Letter, 10 Nov 2011
http://www.nature.com/articles/nature10587.epdf
3. S. Wills, Molecular Machine Power Nobel Chemistry Prize, Optics & Photonics News 5 October 2016
http://www.osa-opn.org/home/newsroom/2016/october/molecular_machines_power_nobel_chemistry_prize/
—H.M. Doss