Passively Cool: A Departure from A/C
January 26, 2015
You’re likely familiar with traditional air conditioning units that can be energy hogs, but a new device could cool buildings with dramatically lower energy expenditures. A team of Stanford engineers has recently developed a device that passively cools to below ambient temperature, even on a sunny California day in direct sunlight!

A view of the sun in ultraviolet on January 1, 2015.
Image Credit: Solar Dynamics Observatory/NASA
Heat Transfer 101
Heat is a type of energy transfer that usually results in objects having a temperature change, and it sometimes results in a material changing phase, like when ice melts or vapor condenses. It turns out that the temperature of an object directly relates to the average kinetic energy (energy of motion) per particle of the object. The change in phase has more to do with the potential energy of the particles or how those particles are situated and attracted to particles nearby.
There are three ways that heat energy can be transferred: conduction, convection, and radiation. To understand conduction, we first must appreciate that molecules are constantly in motion, even in solids. As the molecules move around, they bump into their neighbors. These collisions usually result in a transfer of energy between the colliding particles.
When heat is transferred to the surface of a solid object, the molecules on the surface start to jiggle around more and more. They bump into their neighbors, impart energy to their neighbors, and cause them to move around more. Pretty soon all the atoms in the solid are jiggling around more, and the solid reaches a higher temperature.
The atoms in a solid are “stuck;” they cannot go anywhere they want because they are bound in a lattice (like assigned seats), but they wiggle around that location more and more as they warm up. This process is called heat transfer by conduction. Liquids and gases are not bound in a lattice and can transfer heat energy not only by conduction, but also by convection.
To understand convection, first imagine a pot of water on a hot stove burner. The molecules making up the stove burner are jiggling around a lot and colliding into the molecules of the bottom of the pot. In turn, these molecules at the bottom jiggle around and bump into the molecules above it, and so forth until the inside layer of molecules at the bottom of the pot collide with the water molecules above them.
At this point, some other interesting things happen. Not only do the water molecules begin to jiggle more and bump into their neighbors more, but they also expand a bit, with the hydrogen and oxygen atoms spreading out a littler further from each other.
This causes the water molecules near the bottom of the pot to have a lower density than the water molecules above them, and the lower density molecules rise up as the more dense molecules settle down toward the bottom of the pot. A circulation of material (water molecules in this example) becomes the primary mechanism for heat energy to be transferred throughout the entire pot of water. Certainly, heat transfer by collisions (conduction) also occurs, but it is not the predominant means of transferring the energy throughout the substance.
Finally, Heat can also be transferred by radiation. In this process, electromagnetic radiation, usually in the form of infrared radiation, is emitted from one atom or molecule, and absorbed by another. This absorption of radiation also increases the temperature (the average kinetic energy per particle) of an object.
Net Heat
Considering the rate at which heat energy is transferred to an object minus the rate at which the object transfers heat energy away from it to the surroundings gives a good idea of the resulting temperature the object will sustain. For example, a leaf has heat energy transferred to it at a certain rate from the sunlight shining on whatever part of it is not shaded, but it also radiates away energy to its surroundings from shaded and unshaded surfaces by a number of processes (conduction, convection, radiation, and evaporative cooling).
It turns out to be a very good design. If a leaf did not radiate away any heat energy transferred to it from direct sunlight it could reach temperatures over 1000˚ C in less than 10 minutes. This might remind you of a less extreme situation, a car sitting in a parking lot on a sunny day.
When a car has sunlight shining on it, the visible light easily passes through the windows of the car, which is required for the driver to see anything outside of the car. When the visible light strikes objects that make up the interior of the car, some of it is absorbed by the molecules making up the interior, causing those molecules to have extra internal energy.
Some of this extra internal energy is released from the molecules in the form of infrared radiation. Infrared radiation is an electromagnetic wave we cannot see, but we can “feel” it. These waves are readily absorbed by our skin and cause our skin molecules to move around a bit faster, increasing the temperature. The windows of our car don’t let the infrared radiation out very well, so it gets trapped within the car and builds up.
This happens to buildings also. Sunlight strikes a building, enters it not only through windows, but also by being absorbed by the building and eventually causing increased motion (temperature) of all the molecules on the exterior, which then conduct and radiate heat energy to the interior of the building.
However, if there were a way to let the radiation “out” of the car, or building, then we’d be on the path to cooling. For a sunny place like California this could have a huge impact on energy usage by reducing conventional air conditioning techniques.
Radiative Coolers
Just as the car’s windows have a preference for which radiation they let through (visible light goes through easily but infrared radiation doesn’t go through so well), Earth’s atmosphere also has preferences for which types of electromagnetic radiation can easily pass through, and which types of radiation are more readily absorbed and emitted by the molecules that make up the atmosphere.
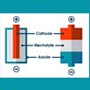
The transmissivity of a material describes the ability for electromagnetic radiation to pass through a material. In the NASA Earth Observatory image below, the atmospheric windows for much of the electromagnetic spectrum are shown.
Percentage of atmospheric transmittance versus wavelength. The near 100% transmittance are “atmospheric windows” that allow wavelengths to propagate through the Earth's atmosphere with nearly zero interaction. Chemical notation (CO2, O3) indicates the gas responsible for blocking sunlight at a particular wavelength. The blue arrow shows the region where there is a large window of transparency in the atmosphere for a range of infrared wavelengths between 8 and about 13 micrometers (µm).
Image Credit: NASA Goddard Space Flight Center
The blue arrow in the diagram indicates a region of infrared radiation wavelengths that have a large transmission percentage through the atmosphere. This set of radiation doesn’t interact very much with the atmosphere and can easily propagate through it. If a material can absorb and hence radiate infrared radiation at these wavelengths, it could readily transfer away heat energy. Generally the atmosphere is more transmittant in low dew conditions (dry days, dry regions, or high altitude).
Earth emits radiation all the time. At night, Earth is able to cool itself to temperatures below atmospheric temperatures because of the windows of transparency in the atmosphere that allow certain radiations to escape the atmosphere to space.
A group of researchers at Stanford recognized this as they thought about what it would take to create a radiative cooler that would cool even under direct sunlight. For such a cooler, they created a photonic solar reflector that reflects up to 97% of incident sunlight and also acts as a very strong thermal absorber/emitter in the region between 8 and 13 micrometers.
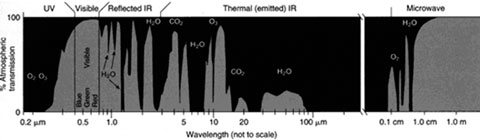
To create this passive radiative cooler the scientists chose to use seven layers of alternating silicon dioxide (SiO2) and hafnium dioxide (HfO2) that rest on a layer of silver (Ag) over a thin (20 nm) layer of titanium to adhere it to a thick silicon (Si) substrate. The thickness of the seven layers was very carefully chosen to maximize the reflectance of the sunlight.
The bottom four alternating layers of SiO2 (a low index material ) and HfO2 (a high index material). The index (index of refraction) describes how well light propagates through the material. The higher the index the slower the light travels through the material. In turn, this slower light is more refracted (bent) and contributes to determining the reflectivity of the surface.
The layers of SiO2 and HfO2 were chosen to be less than 100 nm (as depicted in the diagram) to reflect the most sunlight. The researchers chose HfO2 as a layering material because of its high index, and state in their paper that titanium dioxide (TiO2) could also be used. The alternating layers of high and low index materials and the adjusted thicknesses, as well as HfO2’s low absorption of UV all help to increase reflectance of solar radiation.
The three thick top layers optimize the radiative cooling by various effects. SiO2 strongly absorbs and emits near 9 µm due to a phonon polariton resonance. A phonon is a collective state in a structured material, like a lattice vibration, where all the molecules on the lattice vibrate together in a given way.
A polariton results from combining a photon (packet of light energy) with a collective excited state such as a phonon. In this case an infrared photon couples with a collective vibration state of the crystal, creating a resonance around 9 µm. HfO2, has a nonzero absorption and emission region, over the 8 to 13 µm infrared wavelengths. The combination of optimized layers results in a very strong and selective emissivity between 8 to 13 µm, which coincides beautifully with the atmospheric window mentioned earlier.
Left: Photograph of the radiative cooler on a rooftop in Stanford, CA, on a typical sunny, clear day. Right: a diagram of the seven alternating layers of silicon dioxide (SiO2) and hafnium dioxide (HfO2) atop a 200 nm layer of silver (Ag) with a thin amount of titanium (black) to adhere the silver to a silicon (Si) wafer base. The top three layers are primarily responsible for radiating away energy in the infrared region between 8 and 13 µm. The bottom four layers were optimized for reflecting sunlight.
Image Credit (Left): A. Raman, Stanford University.
Does It Work?
The cooler was tested at various angles and over a number of different days. The scientists found that the device works best on dry clear days, when the atmospheric window is the largest and the most transparent. The results showed that the device on the roof with a 30˚ angle over the time period of 10 AM to 3 PM attains a steady temperature of 4.9 ˚C ± 0.15 ˚C (40.82 ˚F ± 32.27 ˚F) below atmospheric temperature!
If the air is 20˚C (68 ˚F)then the radiative cooler reaches a temperature of about 15.1 ˚C (59.2 ˚F) in direct sunlight. For comparison the researchers considered some common roof materials. Black paint in 20˚C (68 ˚F) ambient conditions reached 80˚C (176 ˚F) in direct sunlight, and aluminum reached about 40˚C (104 ˚F) in the same conditions.
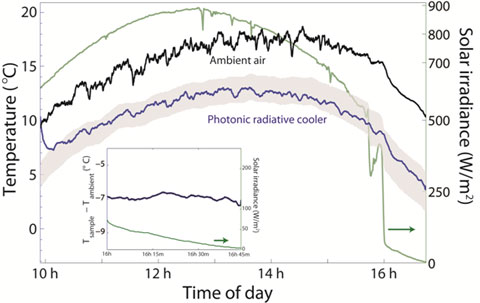
Temperature versus time of day graph showing temperature of radiative cooler and ambient air between 10 AM and 4:45 PM on a clear day in Stanford, CA. The green line is the solar irradiance incident on the photonic radiative cooler. The black line is the measured ambient air temperature, and the blue line is the measured temperature of the radiative cooler.
Image credit: A. Raman, Stanford University.
The researchers developed a theoretical model that agreed well with their experimental results. They used the model to predict and explore steady-state temperatures achieved by the device in different conditions. The model’s predictions include lower steady state temperature values for the device if they can reduce convective losses.
Having such a device as a rooftop, connected to the cooling system of a building, or a car, would be an enormous energy saver because the selective infrared emission and strong reflectance allow for cooling passively without the need for electricity or water (used in evaporative cooling).
What's Next?
The researchers next steps will be to design an efficient way to deliver a heat load to the device so it can radiate that load away. They also plan to minimize any unwanted heating of the device from its surroundings. They are also considering how to integrate the device to chillers (a machine that removes heat from a liquid often by a process of vaporizing and compressing, like many refrigerators) and Heating, Ventilating, and Air Conditioning (HVAC) systems.References and Resources
1. Raman, A.P, et al., Passive radiative cooling below ambient air temperature under direct sunlight, Nature 515, 540–544 (27 November 2014) doi:10.1038/nature138832. NASA Earth Observatory website on Remote Sensing:
http://earthobservatory.nasa.gov/Features/RemoteSensing/remote_04.php
—H.M. Doss