Using Gold Nanoparticles to Kill Cancer
Cancer is an inherently difficult illness to treat. Sometimes residual cancer cells remain even after removing tumors; sometimes parts of a tumor cannot be removed because of the way the cancer cells attach to a vital organ. A way to detect and kill cancer cells in vivo has been sought after for a long time, and some lines of research are finally showing great promise. The latest? Having cancer cells envelop dozens of nanoparticles that then are used to obliterate the cancer cell from the inside, leaving healthy cells untouched!1
The Gist of It
Essentially, nanoparticle therapy works by getting about a hundred gold nanoparticles clustered into a cancer cell, then blasting the area with an infrared laser pulse. Energy imparted by the laser causes the fluid around the cluster to reach temperatures high enough to vaporize the fluid, which causes a rapid expansion and collapse. This results in the obliteration of the cancer cell, but healthy cells are not affected because they don’t incorporate enough gold particles to cause damage. As the high temperatures remain confined within the nanobubble formed around the cluster, nearby cells are unharmed by the process.
How Do You Get Something Inside a Cell?
Cells can incorporate foreign objects in a number of ways, but the method that this research utilizes is called receptor-mediated endocytosis. In this process, the foreign object attaches itself to a point on the outside of the cell called a receptor, which is embedded in the cell’s outer membrane. Receptors have active ends on either side of the cell membrane, each of which attaches to certain molecules; the exterior side is selective for certain particles that the cell needs, while the interior end attaches to the proteins and signaling molecules that regulate cellular processes based on what’s happening outside the cell. The cell uses this method, for example, when it ingests a cholesterol molecule; the molecule binds to a receptor at the cell membrane, leading proteins to attach to the interior end of the receptor, thickening the cell membrane. A pit forms that then envelops the bound molecules into the cell.
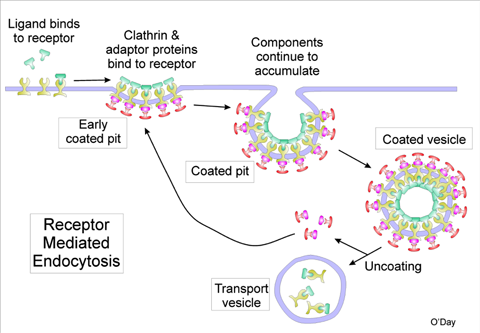
Illustration of receptor–mediated endocytosis.3 Image Credit: Illustration courtesy of Professor Emeritus Danton H. O’Day, Department of Biology, University of Toronto Mississauga, Canada
Preparing the Nanoparticles
For this therapy to be effective, the gold nanoparticles have to attach to a cancer cell more easily than a healthy cell, otherwise the laser pulse would damage healthy tissue. To accomplish this, the researchers coated the particles in an antibody that is known to attach to the specific type of aggressive cancer they were using in the study, head and neck squamous cell carcinoma. It is this antibody that attaches to the receptor at the cell membrane.
The researchers also found that there was an optimal size to the gold particles. If the particles were less then 10 billionths of a meter (10 nanometers) in diameter, the cell would quickly clear them out. If the particles were greater than 100 nanometers the cell had trouble internalizing the particles. The scientists found that the nanoparticles which worked best for their study were around 60 billionths of a meter in diameter.
These antibody-coated gold nanospheres were found to insert themselves into cancer cells far more readily than healthy cells; the average cluster size in healthy cells was found to be 64 nanometers (about 1 sphere), while the average cluster size in cancer cells was found to be about 300 nanometers (about 100 spheres).
One natural advantage to this process is that tumors often have leaky vascular systems, so when the gold particles are injected intravenously near the known cancer, they rapidly spread and are incorporated throughout the cancerous region. The scientists noted that 24 hours of time was needed after the injections to allow gold clusters to form in the cells.
Blowing Up the Cancer Cell
Once the gold nanoparticles are incorporated into cells, the researchers exposed the tissue to a laser pulse (near infrared radiation of wavelength 782 nanometers) for a duration of 30 trillionths of a second (30 picoseconds). This particular type of laser light is optimal because it penetrates tissue well and it is not resonant with the gold nanoparticles. This means that when the light strikes the nanoparticle it does not absorb it and immediately start warming the bulk of the gold nanoparticles resulting in overheating the cell. Rather, during the first 10 nanoseconds some melting of the surface without bulk heating of the gold nanoparticles2 occurs, and this vaporizes the fluid around the gold nanoparticles. The vaporized fluid rapidly expands and then collapses. However, it is imperative to note that the effect is inconsequential unless there are tens of nanoparticles in the cluster. The creation of a nanobubble that rapidly expands and collapses, with enough energy to destroy a cell, is dependent on the number of gold spheres in the cluster, with the severity increasing as the cluster size increases.
This selectivity of severity with size is what keeps the healthy cells safe. The gold nanoparticles don’t do well at transforming the laser pulse to thermal energy on their own, so any nanobubbles formed are relatively insignificant—a few spheres together in a healthy cell will not cause any damage. It is the cluster of nanoparticles within the cancer cells that effectively converts the laser pulse to thermal energy, causing vaporization of the surrounding fluid, a rapid expansion, and a collapse, leading to the destruction of the cancer cell. This event is not easily detected by optical means, but it is easily “heard” by detecting the sound wave produced during the rapid expansion and collapse.
Click here to download a video of the process, directly from the research article!
Taking Out Cancer
This method isn't great at taking out a large tumor, however the researchers have provided some evidence of situations where it may be very useful. If a large number of cancerous cells are removable, then they should be removed surgically, but even after removing a bigger tumor, residual cancer cells can be left behind in the area. These residual cells can grow into tumors, leaving the possibility of the cancer recurring. Detecting single residual cancer cells is not easy, however a cluster as small as three cancer cells can be detected using the gold nanoparticle method. This method then becomes a very useful option for two reasons: 1) detecting residual cancer cells, indicating whether more tissue needs to be removed and 2) using this method to obliterate residual cancer cells. The researchers had success with both methods, resulting in no cancer reappearance in their trials. Removing residual cancer cells with nanoparticles was deemed a new type of surgery by the researchers. They called it “PNB nanosurgery” which stands for Plasmonic NanoBubble nanosurgery1.
There are some situations, when tumors start to grow on essential organs, where the tumor is partially or totally inoperable. In this case, the nanoparticle method has shown promise in destroying enough cancer cells to prolong the patient’s life, albeit not enough to destroy the abundance of cancer cells.
One of the advantages of PNB surgery is that it selectively destroys cancer cells, unlike chemotherapy and radiation therapy. This improves quality of life and makes surgical treatment possible for patients ineligible for other nondiscriminatory treatments1.
Future Research and Development
Future development will be employing PNBs clinically. According the research article, the “surgical algorithms for PNB can be integrated into manual, endoscopic, or robotic surgery by using a standalone PNB probe or by integrating it with a surgical endoscope or robotic arm...”1 The supplementary materials of the research article1 discuss in detail information useful to applying the technique clinically.
References and Resources
1. Lukianova-Hleb, E., et al., Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles, Nat. Nano 343 (2015)
DOI: 10.1038/NNANO.2015.343
http://www.nature.com/nnano/journal/vaop/ncurrent/full/nnano.2015.343.html
2. Lukianova-Hleb, E., et al., Transient Enhancement and Spectral Narrowing of The Photothermal Effect of Plasmonic Nanoparticles Under Pulsed Excitation, Adv. Mater (25), 772-776 (2013)
DOI: 10.1002/adma.201204083
http://onlinelibrary.wiley.com/doi/10.1002/adma.201204083/full
3.O’Day, D., Introduction to the Human Cell—The Unit of Life and Disease, Ebookit, (2012)
https://www.ebookit.com/books/0000001870/Introduction-to-the-Human-Cell.html?HTSAUsite
4. Service, R., Exploding nanobubbles can kill cancer cells, Science, 15 Feb 2016
http://www.sciencemag.org/news/2016/02/exploding-nanobubbles-can-kill-cancer-cells
—H.M. Doss