Natural Reactors
The first controlled nuclear reactor, built during World War II, was a great achievement, but it was not the first reactor to operate on planet Earth. Mother Nature put one together without the benefit of human problem-solving, which only appeared several billion years later.
A typical reactor fuel is the uranium isotope U-235. This isotope has, like all uranium atoms, 92 protons, and its atomic weight—the sum of the numbers of neutrons and protons—is 235. For better or for worse, U-235 is not found in great abundance, making up only about .7% of naturally occurring uranium, and essentially all the rest is U-238. Both U-235 and U-238 spontaneously decay, with half-lives given below.
- Half-life of U-235 = 7.04 x 108 years
- Half-life of U-238 = 4.47 x 109 years
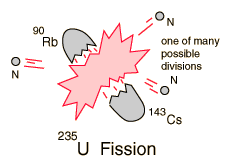
The fission process in U-235. Note the three additional neutrons emitted, which can sustain fission if there are a sufficient number of surrounding U-235 nuclei.
With the passing of each half-life, on the average half of the remaining nuclei decay into nuclei with a lower atomic number by the emission of an alpha particle (a helium nucleus) and gamma rays. This kind of decay is not fission and cannot lead to a chain reaction.
When U-235 absorbs a neutron, there is a high probability that it will promptly fission, with the release of two to three more neutrons, as shown in the drawing. These, in a sufficiently large and concentrated sample of U-235, can set off a chain reaction and release tremendous amounts of energy. A nuclear reactor that sustains a chain reaction is said to be “critical.”
The probability of fission in U-235 depends on the speed of the neutrons, with slow neutrons being much more effective. Substances that cause neutrons to slow down are called moderators, so bathing the uranium in the moderator greatly intensifies the fission process. Water is the moderator of choice for most nuclear reactors, being plentiful and safe and having the added benefit that if the reactor gets out of control and heats up, the water boils off, shutting down the reaction.
Uranium ore contains only about .7% U-235 and is not concentrated enough to sustain a chain reaction without a moderator, so uranium ore must be enriched to a concentration of about 3% to serve as fuel for a natural reactor. In 1956, the Japanese physicist Paul Kuroda realized that, due to the difference in the half-lives of U-235 and U-238 (see above), at earlier times the relative concentration of U-235 would be higher than at present, and this might make possible a naturally-occurring fission reactor. In a remarkable piece of analysis, he detailed the necessary uranium concentration, the relative concentration of U-235/238, and even the reactor shape. Unfortunately, Karuda did not consider the possibility that porous rock might enable a natural water moderator, so he could not identify any uranium mines that matched his requirements
Research
The natural reactor was located at the Oklo uranium mine in Gabon, a country in the southwestern part of Africa (see map). The Oklo mine lies on the site of an ancient river that had concentrated eroded uranium deposits into a rich sludge all along the river bottom. Then, about two billion years ago, blue-green algae evolved, oxygenated the water, and enabled the formation of soluble uranium oxides. These migrated from all over the river into the delta, where reactions in sediments converted them back into insoluble uranium deposits, held in porous sandstone and concentrated in a small area.
The natural reactor was located in Africa, in what is now the country of Gabon, as indicated on the map.

One of the reactor zones at Oklo. Yellow patches are "yellow products" corresponding to recent weathering of uraninite. Photo courtesy of F. Gauthier-Lafaye. CNRS
The first evidence of this reactor, found in 1972, was a slight anomaly in the isotope abundance ratio of U-235 to U-238 in uranium from Oklo (see photo). The observed ratio was .717%, compared to the well-accepted value of .720%. When additional ore samples were analyzed, abundance ratios were found as low as .440%, confirming the U-235 depletion. This significant difference suggested that a nuclear reaction had been at work.
Fission of U-235 produces a well-established array of byproducts, with characteristic relative abundances, which have been carefully measured in the waste produced by nuclear reactors. At Oklo, these byproducts were captured surprisingly well by surrounding mineral grains, and careful analysis showed a match to known fission byproducts with great accuracy, establishing the existence of the natural reactor.
Further measurements provided information about when this natural reactor operated. When U-235 absorbs a neutron, about 20% of the time it does not fission but remains the long-lived isotope U-236, which decays with a half-life of about 2 x 107 years into thorium. Thorium is indeed found at the reactor site, in far greater abundance than elsewhere, whereas U-236 is not found there at all, indicating that the reactor ceased operation only after many half-lives had passed, at least 109 years ago .
How long did the natural reactor operate? Going back to the U-238 that made up 97% of the uranium deposit, multiple neutron capture by this isotope leads to the isotope plutonium 239, which can both fission and decay, with a decay half-life of 24,400 years. Analysis of the Pu-239 byproducts showed that the reactor must have operated for hundreds of thousands of years. Incidentally, the fact that plutonium was produced at Oklo, even two billion years ago, complicates the classification of plutonium as an “artificial element,” i.e., one not found in nature.
What was the moderator? And did the natural reactor operate continuously or in a pulsed mode? These questions were answered by investigators at Washington University. Their detailed analysis of the relative abundance of xenon isotopes, each with a characteristic half-life, indicated pulsed operation and also specified the length of the pulse (30 minutes) and the period between pulses (2.5 hours). Pulsed operation suggests that as the reactor went critical and heated up, it boiled off some of the surrounding water, reducing its moderating effects and stopped the reaction after 30 minutes. Then, after 2.5 hours of cooling, the reaction resumed. Incidentally, these times are typical of geysers, such as those in Yellowstone National Park.
The water moderator limited the reactor to a relatively low power level, to prevent boiling off the water immediately when the reactor went critical. The Oklo reactor operated at a power of about 100 kilowatts, typical of a small research reactor, and in its 150,000 years of operation consumed more than five tons of U-235.
This natural wonder was made possible by a confluence of circumstances that concentrated the U-235, moderated the reaction with a natural control system that prevented meltdown, and preserved the evidence for two billion years. Four of these reactors were found at Oklo, and it’s entirely possible that others existed elsewhere.
Links
Curtin University of Technology, Perth
Washington University in St. Louis
Physics News Update

Another photo of a reactor zone at Oklo. Photo courtesy of F. Gauthier-Lafaye. CNRS