Wave-Particle Duality in One Image
April 21, 2015
Recent observations of both the wave and particle nature of a plasmonic field — a field produced by collectively oscillating electrons on the surface of a metal — in a single image have led to headline news and some misunderstanding. Claims about the possible upending of the principle of complementarity, which states that one cannot simultaneously observe both wave and particle behavior, popped up in the media recently. But don’t worry; the principle of complementarity remains upheld.
Wave-Particle Duality
In the early 1900’s, quantum physics developed out of findings that electromagnetic waves could behave like particles when striking the surface of a metal and ejecting an electron. If it behaved only as a wave, then increasing the amplitude of the wave would increase the speed of the ejected electron. But rather than increasing the speed of the electron, the increased amplitude just increased the number of electrons ejected.
Physicists also found that the frequency of the light wave interacting with the metal surface played an important role. If the frequency of the electromagnetic wave was below a certain value (which depended on the type of metal), then no electrons were ejected. If the frequency of light was continually increased above the minimum value, then the ejected electrons had greater kinetic energy.
Einstein proposed in 1905 that the electromagnetic wave striking the metal surface behaved as a particle called a photon. A photon is a localized unit, or packet, of electromagnetic energy. The energy a photon carries equals its frequency multiplied by a constant, called Planck’s constant. (Planck had introduced this value a few years earlier while trying to fit blackbody radiation data).
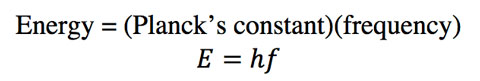
When the photon struck the metal surface, it ceased to exist, transferring its energy to the electron. If the photon had the minimum frequency needed for the electron to be ejected, then the electron was ejected but had no motion upon ejection. If the photon had a greater frequency (and hence a greater energy), the electron was ejected and had motion, which could be measured. The amount of energy associated with the electron’s motion was equivalent to the energy the photon had above the minimum required amount to eject an electron.
Physicists built greatly upon Einstein’s ideas in the next few years, including the Bohr model introduced in 1913 by Niels Bohr. He developed a quantized model of the hydrogen atom that had electrons orbiting a nucleus at specific values of radii and corresponding energy. This model helped to explain emission spectra that were observed at the time. Transitions from an upper energy level to a lower energy level corresponded to the the emission of a photon whose energy was equal to the difference in energies between the two levels.
About twenty years after Einstein introduced the idea of a particle nature associated with an electromagnetic wave, de Broglie introduced the idea of a wave nature associated with a particle. Because electromagnetic waves can act like particles and have momentum (described also by the special theory of relativity), de Broglie suggested in 1924 that particles might act as waves.
The relationship between the momentum of a photon and its wavelength is:

de Broglie introduced the idea that the wavelength describing a particle’s wave nature is
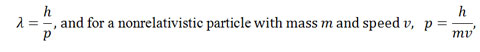
Nonrelativistic particles are particles moving at speeds not near the speed of light. The momentum of a nonrelativistic particle is its mass multiplied by its velocity. For an everyday item such as a car (about 1800 kg) moving at 65 miles per hour (about 30 m/s), the wavelength would be 1.227×10-38 meters, which is too small to be measured. For an electron traveling at one quarter the speed of light it would be 9.698×10-12 m, a small, but measurable effect.
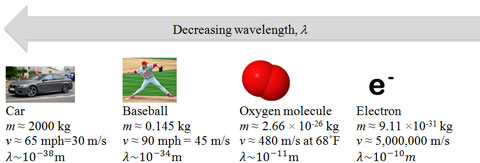
Image Credits: Alexandre Prevot, SD Dirk, and Ulflund, respectively from left to right via Wikimedia Commons.
The History of Wave-Particle Duality
Since the introduction of the ideas that waves can behave as particles and particles as waves, there have been an incredible number of experiments that support these ideas, and no experiments have presented a serious problem with them.
A classic example is the double-slit experiment — monochromatic coherent light shining through two slits - which produces an interference pattern at a distant screen, does so also for particles, as long as we don’t try to determine which slit the particle went through.
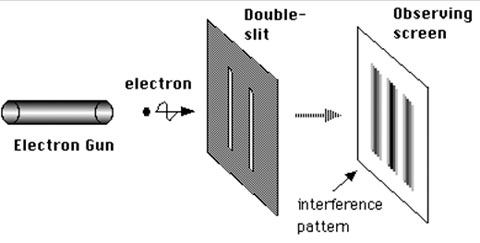
Diagram of double slit experiment using electrons.
Image Credit: NekoJaNekoJa via Wikimedia Commons
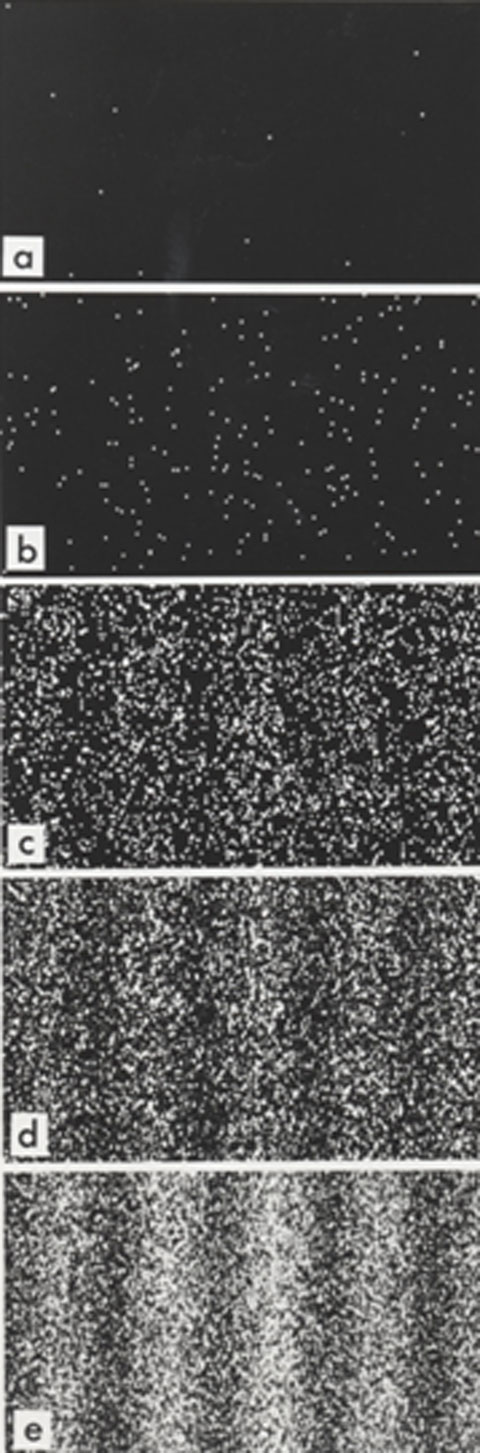
Results of a double-slit-experiment performed by Dr. Tonomura showing the build-up of an interference pattern of single electrons. Numbers of electrons are 11 (a), 200 (b), 6000 (c), 40000 (d), 140000 (e)
Image Credit: Belsazar via Wikimedia Commons
Complementarity Upheld?
We need both wave and particle descriptions to describe completely a photon or electron. but as the double-slit experiment demonstrates, we are unable to simultaneously measure both behaviors. If we try to measure the wave behavior then we only see wave behavior, and if we try to measure the particle behavior then we only see the particle behavior. This is known as the principle of complementarity.
The image below is a big deal. Never before has a single image been taken that captures both wave- and particle-like behavior. However, this is not capturing wave and particle nature of a single photon or single electron, rather it captures the wave as the field exchanges photons (energy packets) with a beam of electrons being used to image the field.
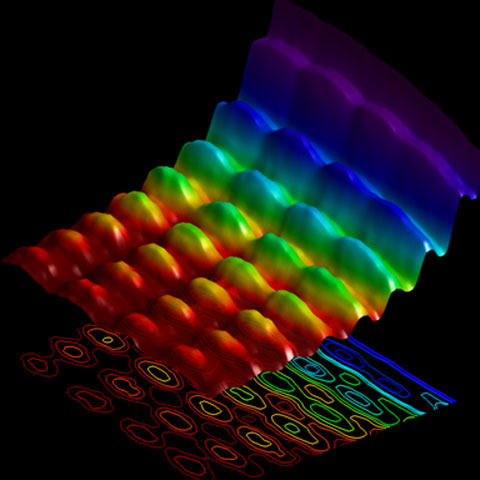
The lower portion of the image shows the projection of the 3D map above it. Quantization can be seen along the x-axis, while the wave pattern appears along the y-axis.
Image Credit: Fabrizio Carbone, EPFL
A special electric field, known as a surface plasmon polariton (SPP) field, is confined to the surface of a metal nanowire. The field cannot move perpendicular to the surface, but can move along the surface, and therefore it can interfere with reflected fields off of the ends of the nanowire to produce a standing wave pattern, much like a resonant wave on a plucked string.
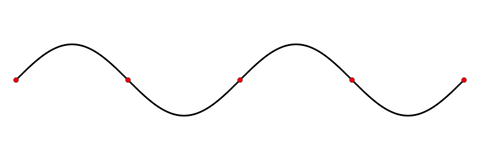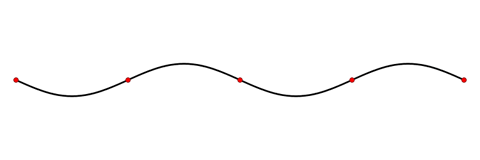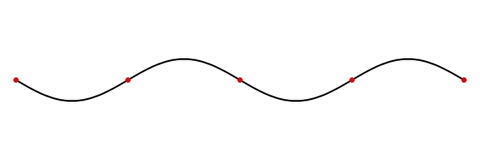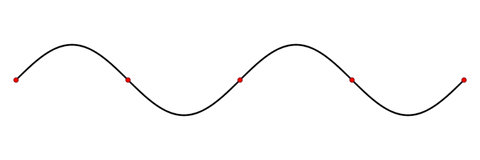
A standing wave.
Image Credit: LucasVB via Wikimedia Commons
The surface field is produced by the collective oscillations of the free electrons on the metal surface. Standing wave patterns (resonant patterns) are induced by sending a pulse of 800 nm infrared (IR) laser light.
The pulsed laser light sets the collective oscillation of electrons on the surface (called a surface plasmon) and a surface plasmon polariton surface field is created. The term polariton is used to define the field’s strong coupling to a dipolar excitation, which is due to the distribution of electrons in the metal nanowire. A resonant mode is obtained by adjusting the polarization (direction of electric field) of the pulsed infrared laser.
To image the surface field, the scientists probe it with electrons. The electrons come from an electron gun, which utilizes the photoelectric effect mentioned earlier. A pulsed ultraviolet (UV) laser strikes a metal surface, ejecting bunches of electrons with relativistic speeds.
The electrons are detected and their change in energy is measured. The scientists used a new method for imaging the field called Photon Induced Near-field Electron Microscopy (PINEM) and utilized an ultrafast energy-filtered transmission electron microscope.
By measuring how the electron’s energy is affected, the scientists observed how the field interacted with the electrons. In this way they can image the field. If the electrons speed up, then energy was passed to the electrons from the field and if they slowed down then energy was transferred from the electrons to the field. By filtering out only electrons that sped up and imaging how many electrons came from a given location around the nanowire for various time delays, they mapped out how the standing wave pattern evolved over time on the surface of the nanowire.
The standing wave is formed after the IR pulse reaches it, then the peaks and troughs of a standing wave pattern oscillate back and forth. The nodes, however, remain stationary, and eventually the standing wave dissipates. As the electrons approach the surface field, they get accelerated toward or away from the field depending on the field’s direction at that instant.
If the electrons are speeding up toward the field as they head toward the detector, then there will be a higher electron count coming from that location than a location they are not being pulled toward. This provides information about the spatial variation of the standing wave on the nanowire allowing for imaging of the wave nature of the field on the wire.
The researchers have a method to control the amount of time between creating the resonant field on the metal nanowire and interaction between the resonant field and the electrons. They do this by adjusting the time between the IR pulse (that creates the resonant field) and the UV pulse (that ejects the electron bunches).
This allows them to observe in time how the resonant field evolves on the surface of the metal nanowire, noting that nodes of a resonant wave stay nodes in time, and peaks turn into valleys and back to peaks again. The delay time can be controlled to within femtoseconds (trillionths of a second).
As a comparison, our modern day movies usually show around 25 frames per second. In contrast, these scientists were viewing about two trillion frames per second, but keep in mind that the entire process occurs over times much less than a second.
When an electron interacts with an electromagnetic field, it exchanges quantized packets of energy. The results of the experiment show a discrete absorption and emission spectra when the time delay is set to zero. The scientists measured an energy quanta of 1.55 eV, and integer multiples of that energy amount (See Ref 1. Fig. 1).
By filtering out the electrons to only view electrons increasing in energy (gaining energy packets), the scientists were able to map out when the field exchanged an energy packet (photon) with the electron. By overlapping the spatial electron intensity information for a zero time delay with the energy packet exchange information, the scientists were able to capture both the wave nature of the surface field and its particle nature exhibited by its exchange of photons with the imaging electrons.
Meanwhile, the experiment followed the rules as we understand them of quantum mechanics by not observing simultaneously the wave and particle nature of any single photon.
Below is a video created by École polytechnique fédérale de Lausanne (EPFL), that presents this information in another way.
Future Work
The scientists are now working on filming plasmonic interferences. They are trying to see the limit of the complementarity principle by looking at single electron single photon exchanges.
References and Resources
1. Piazza, L., et al., Simultaneous observation of the quantization and the interference pattern of a plasmonic near-field, 6:6407 doi: 10.1038/ncomms7407 (2015).http://www.nature.com/ncomms/2015/150302/ncomms7407/full/ncomms7407.html
2. Stein, B., No, You Cannot Catch An Individual Photon Acting Simultaneously As A Pure Particle And Wave, Inside Science 13 March 2015
http://www.insidescience.org/blog/2015/03/13/no-you-cannot-catch-individual-photon-acting-simultaneously-pure-particle-and-wave
—H.M. Doss