Imagine Better Imaging with Antimatter
Ordinary matter — the type comprising the earth, our bodies, and much of the visible universe — is made up of three kinds of particles: protons, neutrons, and electrons. Protons and neutrons are the heavy bits that make up the center (nucleus) of an atom, while the much lighter electrons form a cloud around it. A proton and an electron have equal but opposite charge, so when there are the same number of protons and electrons in an atom, it’s neutral and stable.
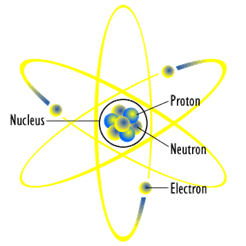
Each of these particles, though, has a sort of “evil twin” — its antiparticle. For instance, a proton’s antiparticle, simply enough, is called an anti-proton, and an electron’s antiparticle is called a positron. A positron weighs the same as an electron, but it has the same charge as a proton. Since opposite charges attract, electrons are drawn to positrons the same way they are to protons. If a particle ever meets its antiparticle, though, something amazing happens — they completely destroy each other! Scientists call this annihilation, and even though it sounds frightening, you’d have a hard time collecting enough antimatter for it to be dangerous — virtually the only time we see antimatter is when we make it in the lab.
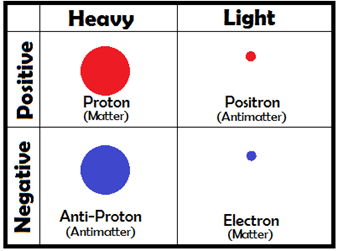
This annihilation CAN be useful, though! When two particles annihilate each other, they don’t just vanish without a trace. Their charges cancel out, but the energy that they contained has to go somewhere. This has a lot to do with the most famous equation in modern physics:
E=M×c2
Here, E stands for Energy, M for Mass, and c for the speed of light. When a particle annihilates its antiparticle, they both turn into photons, rays of light, which shoot off in opposite directions. These photons have a very specific frequency, which we can figure out using a formula known as Planck’s Law:
E=h×ν
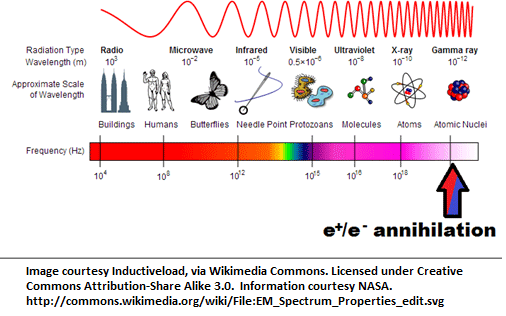
Here, E is the rest-energy of the electron, h is Planck’s Constant, and ν is the frequency.
But what does this have to do with imaging?
Under the right conditions, certain elements can turn into other elements. 18F, fluorine-18, is one of them. 18F is made by shooting high-energy proton beams at oxygen in a particle accelerator. Over the next few hours, the fluorine atoms turn back into oxygen and, when they do, they each emit a single positron!
In the 1950s, scientists realized that we could use this property to look inside someone with much greater detail than X-rays provide and, in turn, to track down diseases like cancer. To do this, doctors attach Fluorine-18 to sugar molecules, and feed them to a patient. The sugar makes its way through the patient’s body, getting absorbed into various tissues. Tumors take in sugar at a very high rate, so if the doctors find a lot of this radioactive sugar where they weren’t expecting to, it can alert them to potential tumor sites.
How do they see it?
When the fluorine-18 lets off a single positron into the swarm of electrons in your body, it gets annihilated almost instantly. As we mentioned earlier, annihilation results in gamma rays with a very specific energy. The same way you can tune a car antenna to pick up a certain energy (frequency) radio wave, scientists can look for these gamma rays coming out of a person. They pass through flesh and bone, so by putting the patient in a special imaging chamber, we can catch all the light that comes from those annihilations.
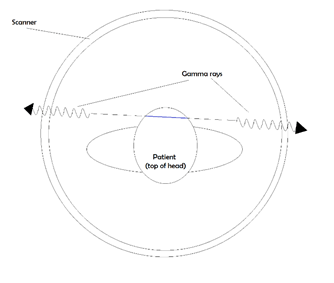
By looking for photons that come out in opposite directions at the same time, and then tracing back their paths, we can figure out where they started. In the image above, catching the two photons illustrated tells the detector that there was a positron somewhere along that blue line. By finding a lot of these lines and looking at where they cross each other, we can reconstruct an image of the patient, inside and out!












