The Circle Game
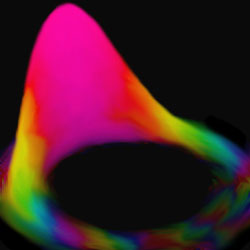
In spite of the uncertainties of quantum mechanics, an electron can orbit the atomic nucleus like a planet orbiting the sun (an orbiting electron's quantum "wave packet" is shown here). Experiments demonstrated the effect more clearly than ever before. Image Credit: Animation courtesy of Carlos Stroud, University of Rochester
Computer simulations show the spreading of an electron's wave packet as it orbits a nucleus. More explanation is available here. In the experiments described above, the single-hump wave packet survived much longer than it does in this animation. (2.8 MB)
Like a planet orbiting the sun, some ideas keep coming around. In the 1920s, the inventors of quantum mechanics scuttled the notion that an atom behaves like a tiny solar system. Whereas the planets orbit the sun in elliptical orbits, the electrons hover around the nucleus in diffuse cloud-like waves, known as orbitals, that describe only the probability of finding the electron at different places within the atom. But those orbitals can combine to form a clump-like "wave packet" that contains the electrons and does orbit the nucleus like a planet, researchers reported in April 2004. The observation confirms a long-sought connection between the quantum and classical realms.
Read more about this research at Physical Review Focus.
Text courtesy of Physical Review Focus.











