Nanostructures Promise Iceproof Surfaces
February 27, 2018
Frost Formation Stopped Cold
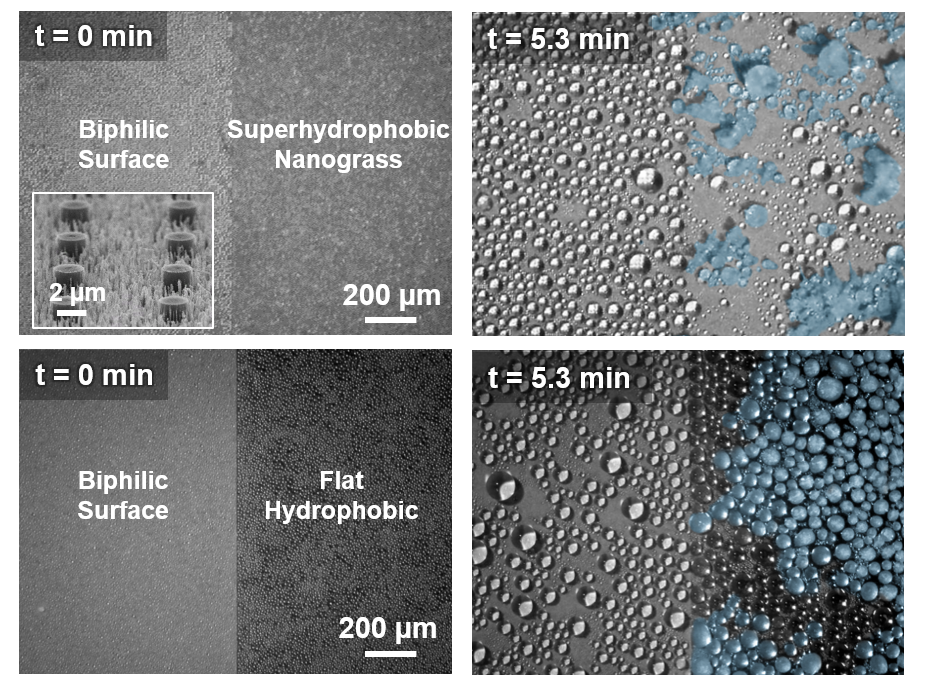
What's Going On Here?
Frost and ice are two things you'd certainly expect to find in a freezer, but when they're unwanted they can present huge problems. Ice is a great insulator, which means that an object placed in a frost-encrusted freezer will get cold at a much slower rate than it would if the walls were bare metal. This might not be an issue in dry environments, but ambient humidity—water vapor dissolved in the air—can condense on cold surfaces, like breath fogging up a window in wintertime. When the tiny droplets of water in that fog freeze, it can build up into a frost coating that ruins the freezer's efficiency.
One of the most popular methods to try and solve this problem has involved using superhydrophobic (water repellent) surfaces; if the surface can prevent water from forming on it, the logic goes that it might be able to prevent icing as well. However, so far this technique has only found limited success.
For water droplets to roll off of something, they have to grow large enough that the force of gravity overcomes their adhesion to the surface. Superhydrophobic surfaces work to reduce that adhesion, but the droplets that form a condensation "fog" are extremely tiny, so they freeze easily before reaching a large enough size to roll off.
There's another way for droplets to leave a surface, though—they can jump! When two neighboring droplets grow large enough to touch and join together, the force of this coalescence can propel them off the surface!
By interspersing tiny hydrophilic (water-attracting) “pillars” among the nano-grass of a superhydrophobic surface, as shown in the top-left inset image, it’s possible to create spots where the droplets will grow large enough to “jump” off before freezing. By taking advantage of these jumping-off points, we may be able to design frost-proof surfaces!














