Defining Time
June 6, 2016
If you’ve ever bid on a popular item during an online auction, stopped at the store just after closing, or stared, starving, into a microwave for the final countdown—you know the value of a second.
But what exactly is a second? If the power goes out for days and all the cell phone batteries die, who knows what time it is when the power comes back on? If two things happen at almost exactly the same time, at what point can we stop measuring which happened first?

The launch of Space Shuttle Discovery on July 4, 2006. The countdown clock on the grounds of the NASA news center shows 7 seconds into the launch.
Image Credit: NASA/George Shelton. Public Domain.
Like the pound, kilogram, inch, and meter, the second is a unit that people use all the time, but most of us never give a thought to who defines these units and how. Thankfully we don’t have to. Consider what would happen if you need a 3-foot board for a project and the clerk at the lumberyard used his foot to measure? Having units that are standard—widely adopted and precisely defined—is essential for commerce, communication, safety, and sanity. Our whole way of life would collapse without metrology, the science of weights and measurement.
This idea is so important that, in 1875, several nations signed the Treaty of the Metre to create a system for developing and overseeing units of length and mass. This was later expanded to include all units within the current International System of Units, or SI for short. This includes, among others, the meter, kilogram, and second.
The second hand debuted on clocks about 350 years ago, representing 1/60th of a minute. As a standard unit of time, however, minutes and hours are now based on the second instead of the other way around.
The first official definitions for the second were based on our relationship to the sun—a second is a fraction of a minute, which is 1/60th of an hour, which is 1/24th of how long it takes the earth to rotate once. Today, the definition is based on the behavior of electrons in cesium atoms, and recent research suggests that we are on the verge of an even more precise definition.
The second can sometimes seem like the penny—an annoying extra that we usually round up or down from anyway. But in today’s high-tech world of rapid high-value stock trades and high-speed communication, tiny fractions of a second can make all the difference. As technology grows at a rapid pace, our need to timestamp events accurately and precisely does too.
Defining a Second
You can keep time with anything consistent and repetitive, like the swinging pendulum of a grandfather clock, a steady drumbeat, or a flashing light. To keep time accurately over a long period, the “ticks” must be evenly spaced and continue for a long time. If you want to describe the time precisely, the ticks need to be close together. They must not be affected by external conditions temperature, humidity, altitude, or the clock’s distance away from you. If you want people in different locations to use the same kind of clock, you have to be able to create others that behave exactly the same way as yours.
Now add in the requirement that your clock needs to be precise enough to handle financial transactions, the needs of the electrical grid, and GPS systems, and there aren’t many options.
In the 1940s, Isidor Rabi, a physicist at Columbia University, suggested that the behavior of electrons in an atom could be a more reliable and precise way to measure time than using our relationship to the sun. The first atomic clock was built at the National Institute for Standards and Technology in 1949. The designs have been refined, but the basic idea remains the same.
Atoms are composed of a central nucleus, surrounded by a cloud of electrons.
Electrons can be in different “states” depending on their energy. Give an electron some energy, maybe by shining a laser on it, and it can jump to a higher, excited state for a short time. Then, before too long, the electron will drop back to the ground state and give off the same amount of energy. This moving from one state to another is called a transition, and the energy it takes is called the transition energy. Many atomic clocks are based on the transition energy of a specific transition that occurs in cesium atoms.
The transition energy is in the form of electromagnetic radiation, or light, from the microwave part of the spectrum. You can describe electromagnetic radiation by its frequency. Frequency is an inverse measurement of time—it tells you how many times something happens per second. In the case of light, it tells you how many cycles of the light wave go by per second.
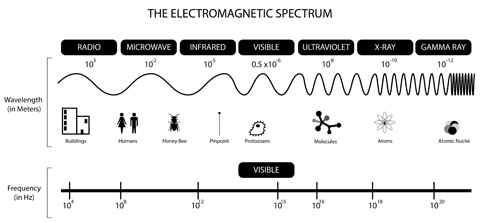
Infographic of the electromagnetic spectrum.
Image Credit: Jonathan S. Urie (CC 3.0)
Cesium clocks are based on a transition with an energy of 9,192,631,770 Hertz (cycles per second). Every time this transition happens in a cesium atom, that same amount of energy is absorbed or released.
The current definition of a second is the time that it takes for 9,192,631,770 cycles of the radiation that correspond to this transition. Scientists measure this using sophisticated clocks built in labs that don’t look anything like polished, commercial clocks. This definition is so precise because you can divide one second up into 9,192,631,770 smaller time intervals.
The official time, called International Atomic Time (TAI), is calculated by a kind of averaging of the times from around 500 networked cesium fountain clocks (“fountain” refers to a particular clock design). The clocks are located in labs around the world. In the United States, the standard time is kept by NIST-F2 at the National Institute of Standards and Technology (NIST) in Boulder, Colorado. If NIST-F2 ran continuously for 300 million years, it would still be accurate to within one second! TAI is closely related to Coordinated Universal Time (UTC), which you may be more familiar with since time zones are based on UTC.

NIST physicists Steve Jefferts (foreground) and Tom Heavner with the NIST-F2 cesium fountain atomic clock, a new civilian time standard for the United States.
Image Credit: NIST. Public Domain.
A New Candidate for the Second
This definition has served us well since 1967, but another change may be on the horizon.
A different kind of atomic clock based on strontium instead of cesium is gaining momentum. New research shows that a network of clocks based on a particular design, called strontium optical lattice clocks, could measure time even more precisely and keep time even more accurately than we can today.
Although they have a different design, strontium clocks are based on the same fundamental idea as cesium clocks. They are nicknamed optical clocks because the transition energy that makes them tick is in the optical, or visible, part of the spectrum—not the microwave part like cesium clocks. Optical clocks can measure time more precisely because the transition energy has a frequency about 100,000 times higher, which means there are more ticks per second.
Optical clocks have been around for a while, but they are technically complicated and have long downtimes. Scientists thought that they weren’t very good candidates for defining a second for this reason. Until now.
Experiments by researchers at the national metrology institute in Germany, Physikalisch-Technische Bundesanstalt, recently demonstrated a system based on an optical clock that performed significantly better than the best cesium fountain clocks over a period of 25 days. Their research shows that in the long run, a network of optical atomic clocks would more stable and reliable than the existing network, even with the downtimes.
The definition of a second isn’t likely to change this second though. Optical clocks are advancing rapidly and there are several designs. Which one will win out and give us the most options for whatever wild advances the future brings remains to be seen.
—Kendra Redmond













